Microglia are central to pathogenesis in many neurological conditions. Drugstargeting colony-stimulating factor-1 receptor (CSF1R) to block microglialproliferation in preclinical disease models have shown mixed outcomes, thusthe therapeutic potential of this approach remains unclear. Here, we show thatCSF1R inhibitors given by multiple dosing paradigms in the Tg2541 tauopathymouse model cause a sex-independent reduction in pathogenic tau andreversion of non-microglial gene expression patterns toward a normal wildtype signature. Despite greater drug exposure in male mice, only female micehave functional rescue and extended survival. A dose-dependent upregulationof immediate early genes and neurotransmitter dysregulation are observed inthe brains of male mice only, indicating that excitotoxicity may precludefunctional benefits. Drug-resilient microglia in male mice exhibit morpholo-gical and gene expression patterns consistent with increased neuroin-flammatory signaling, suggesting a mechanistic basis for sex-specificexcitotoxicity. Complete microglial ablation is neither required nor desirablefor neuroprotection and therapeutics targeting microglia must consider sex-dependent effects.
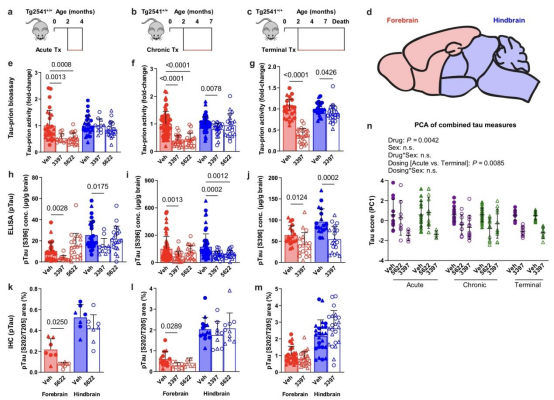
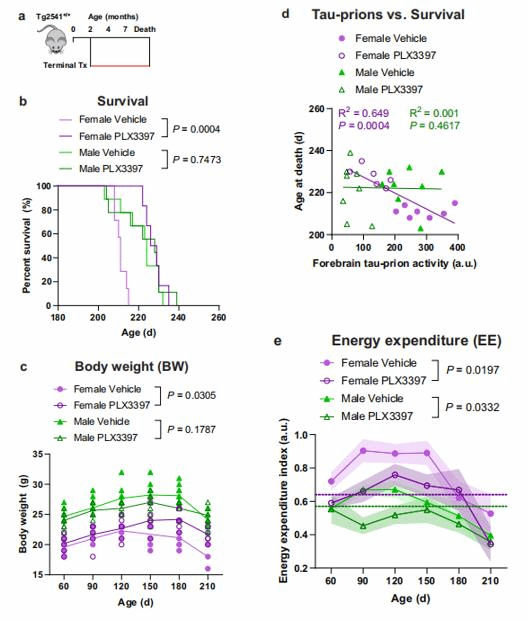

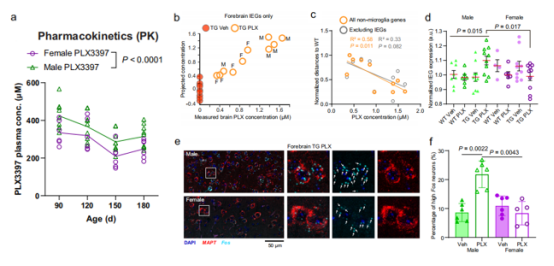
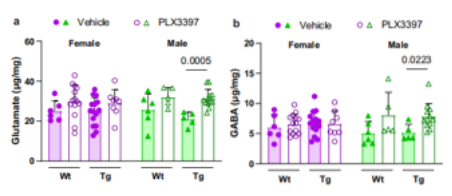
paper link: https://www.nature.com/articles/s41467-022-35753-w