On November 14, 2023, Prof. Wei-Guang Li from our institute, together with Prof. Fei Li's team at Xinhua Hospital affiliated with Shanghai Jiao Tong University School of Medicine, and Prof. Qiang Luo's team from the Institute of Brain-inspired Intelligence Science and Technology at Fudan University, published a research article online in the journal Research, jointly established by the China Association for Science and Technology (CAST) and the American Association for the Advancement of Science (AAAS). The paper, entitled "SH2B1 Tunes Hippocampal ERK Signaling to Influence Fluid Intelligence in Humans and Mice," integrates multidimensional population-level data analysis, mouse models, behavioral assays, magnetic resonance imaging (MRI), and single-cell RNA sequencing to reveal that the obesity risk gene SH2B1 regulates intelligence directly via its action in the hippocampus, independently of its metabolic roles. Further mechanistic studies in mice demonstrated that SH2B1 modulates cognitive functions through hippocampal extracellular signal-regulated kinase (ERK) signaling, providing important theoretical insights into how obesity-associated genes impact cognition and intelligence.
In psychology, the macroscopic manifestations of intelligence (such as IQ test scores) remain largely disconnected from the underlying microscopic neural mechanisms, highlighting an urgent need to bridge this gap. By taking human behavioral genetics data as an entry point and examining evolutionary conservation across species, scientists aim to construct a comprehensive model of intelligence to facilitate its understanding and enhancement. Previous studies have revealed associations between intelligence and metabolic indicators, yet the nature of this relationship—sequential or parallel—remains unclear. Under pathological conditions, Alzheimer's disease, which is characterized by declining cognitive function, is often accompanied by insulin resistance and impaired glucose regulation in the brain, earning it the designation "Type 3 diabetes". Nevertheless, the broader relationship between intelligence and metabolism remains poorly understood.
Focusing on the biological basis of fluid intelligence—a heritable cognitive capability independent of acquired knowledge—the research team first performed a genome-wide association study (GWAS) using data from the UK Biobank cohort. They discovered that three single-nucleotide polymorphisms (SNPs: rs4788102, rs7498665, and rs7359397) in the obesity risk gene SH2B1 were not only significantly associated with body mass index (BMI) and trunk fat mass (TFM), but also robustly linked to fluid intelligence (Fig. 1). Intriguingly, the association between SH2B1 polymorphisms and fluid intelligence remained significant even after adjusting for BMI, TFM, and other metabolic indicators.
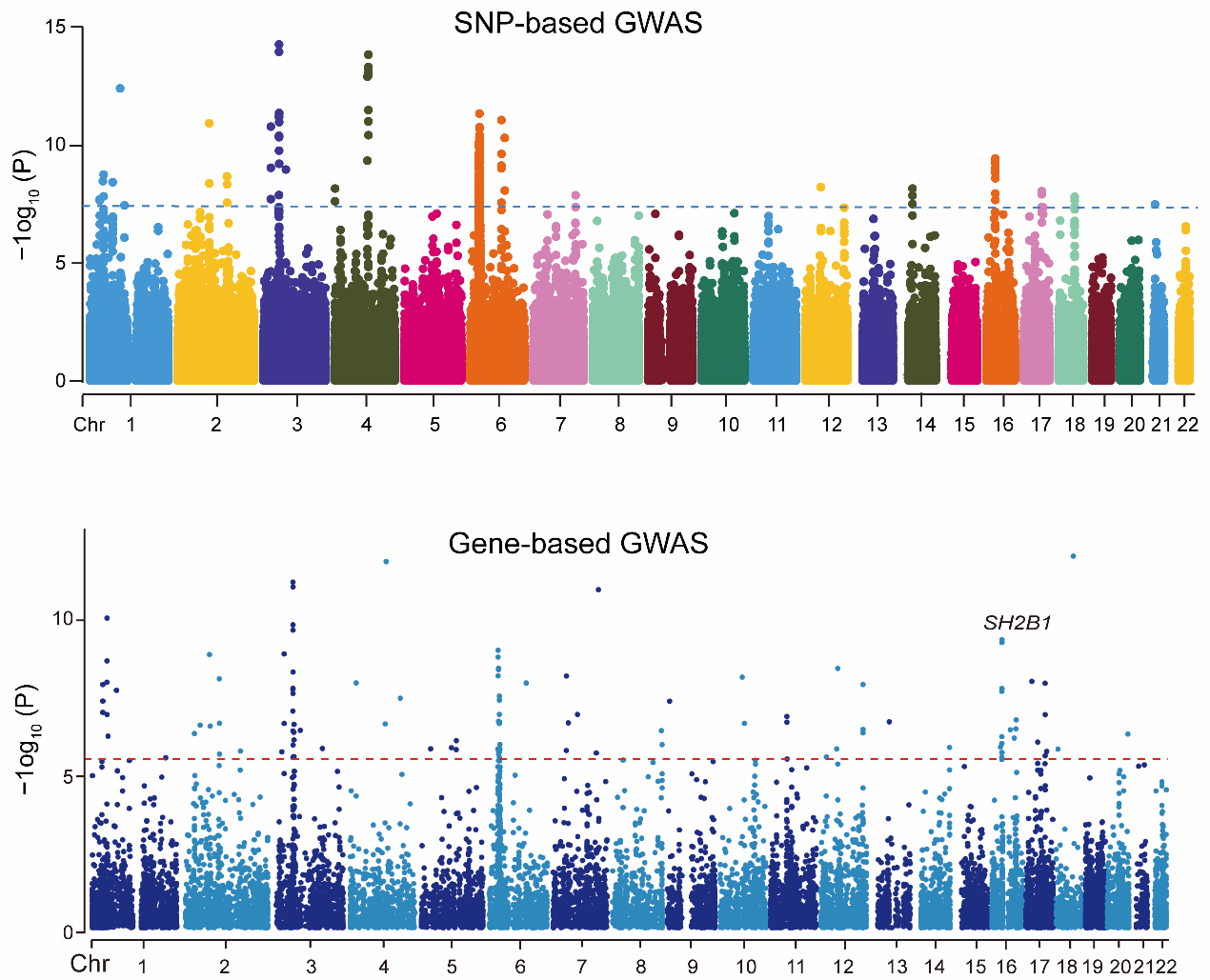
Figure 1. Genome-wide association study (GWAS) of fluid intelligence in the human population.
The researchers further observed correlations between the three SH2B1 SNPs and additional behavioral factors such as alcohol consumption and physical activity. After controlling for these covariates (including alcohol frequency, hip circumference, total body fat mass, neuralgia, and recent physical activity type), the genetic association between SH2B1 variants and fluid intelligence remained significant. Given the known genetic correlation between education level and intelligence, researchers also adjusted for educational attainment, and the SH2B1-fluid intelligence association persisted strongly and independently, underscoring the robustness of this genetic link irrespective of educational factors.
By integrating human genetic data, neuroimaging, and cognitive behavioral analyses, the study further revealed significant associations of SH2B1 genotype with hippocampal volume and functional connectivity within the default-mode network (DMN) and frontoparietal network. Mediation analyses specifically showed that hippocampal CA1 region volume mediated the effect of SH2B1 variants on fluid intelligence independently of BMI, suggesting a direct pathway through which SH2B1 regulates intelligence via hippocampal structure and function (Fig. 2).
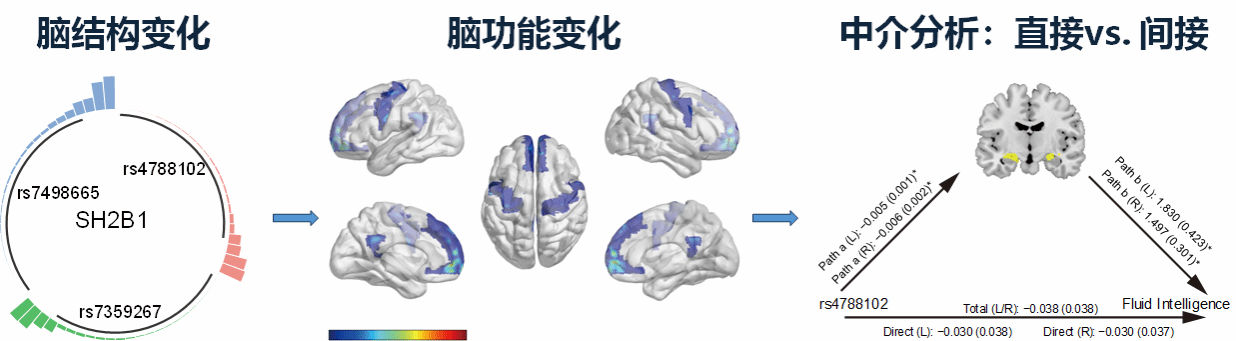
Figure 2. SH2B1 gene polymorphisms mediate human intelligence performance by regulating hippocampal volume.
To elucidate the neural mechanisms underlying SH2B1's effects on intelligence-related behaviors, the team employed mouse models. Selective deletion of Sh2b1 specifically in the hippocampal CA1 region resulted in impaired working memory, short-term memory, and cognitive flexibility, while long-term memory, basal emotional responses, locomotor activity, and metabolic parameters remained unaffected (Fig. 3). Mechanistic investigations revealed that SH2B1 influences cognitive performance selectively through GABAergic inhibitory interneurons rather than excitatory glutamatergic neurons. Single-cell RNA sequencing further demonstrated that Sh2b1 deletion in inhibitory interneurons markedly elevated ERK signaling, and pharmacological blockade of this signaling pathway rescued the behavioral deficits. These cross-species findings indicate a conserved link between SH2B1-related metabolic risk variants, neural circuitry, and cognitive functions, offering meaningful parallels between mouse cognition and human intelligence measures.
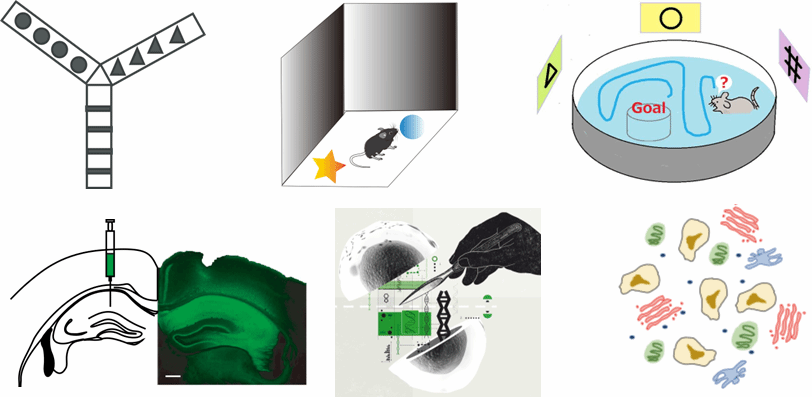
Figure 3. Analysis of neural mechanisms underlying Sh2b1-mediated regulation of intelligence-related cognitive behaviors in mice.
In summary, this study elucidates conserved genetic and neural mechanisms by which the metabolic risk gene SH2B1 influences intelligence-related behaviors in both humans and mice. By bridging genetic variations, metabolic pathways, and cognitive neuroscience, this work significantly advances our understanding of the complex relationship between intelligence and metabolism. These findings not only expand our appreciation of gene-cognition-metabolism interactions but also provide valuable clues for future studies on the diagnosis and treatment of diseases characterized by combined metabolic and cognitive impairments.
Authorship and Acknowledgments:
Dr. Xiujuan Du from the Department of Developmental and Behavioral Pediatrics at Xinhua Hospital affiliated with Shanghai Jiao Tong University School of Medicine, postdoctoral fellow Dr. Yuhua Yan from our institute, and Prof. Juehua Yu from Kunming Medical University are co-first authors. Prof. Fei Li (Xinhua Hospital affiliated with Shanghai Jiao Tong University School of Medicine), Prof. Qiang Luo (Institute of Brain-inspired Intelligence Science and Technology at Fudan University), and Prof. Wei-Guang Li from our institute are co-corresponding authors.
This study was supported by various programs including the National Key Research and Development Program of China, National Natural Science Foundation of China, Shanghai Science and Technology Commission, Shanghai Municipal Health Commission, and Shanghai Municipal Education Commission. The authors also acknowledge collaborative support from Prof. Jianfeng Feng and Prof. Xiao-Yong Zhang at Fudan University's Institute of Brain-inspired Intelligence Science and Technology, Prof. Liangyou Rui from the University of Michigan, Prof. Longnian Lin from East China Normal University, and Academician Shumin Duan from our institute.
Link to original paper: https://spj.science.org/doi/10.34133/research.0269