Depression is a common mental disorder that severely restricts social and psychological functions, reduces quality of life, and imposes a huge burden on health and economy [1]. The pathophysiological mechanism of depression is complex and needs to be fully elucidated [2], while the current clinical treatment of depression has problems such as low efficacy and obvious side effects [3, 4]. Therefore, further research on the molecular pathology of depression and the search for new treatment methods are of great significance.
The nutritional components, including amino acids, play a crucial role in the influence on depression-related behaviors [5,6]. Among them, the research on the regulation of depressive-like behaviors by amino acids mainly focuses on the functions of amino acids and their metabolites as neurotransmitters [5,6]. However, the research on amino acids as signaling molecules that regulate neuronal functions and depression-related behaviors is still scarce. Currently, some essential amino acid supplements are believed to help improve depressive behaviors [10,11], but does the deficiency of a certain essential amino acid have an impact on depressive behaviors? On February 4, 2023, the Guo Feifan team from the Institute for Translational Brain Research of Fudan University published a research paper titled "Leucine deprivation results in antidepressant effects via GCN2 in AgRP neurons" in the journal Life Metabolism. The authors used a chronic restraint stress (CRS) depression mouse model and found that a short-term essential amino acid deprivation diet, the leucine deprivation diet, could significantly improve the depressive-like behaviors induced by CRS and had no effect on non-stressed mice. This beneficial effect was not related to food intake and was applicable to other essential amino acid deprivation diets. In addition, they found that the hypothalamic AgRP neurons were involved in the process of the leucine deprivation improving the depressive-like behaviors induced by CRS, and the amino acid sensor general regulatory repressor protein kinase 2 (GCN2) mediated this behavioral effect in AgRP neurons.

The same issue of the magazine also published an article titled "You are what you eat: Feeding Neurons in Nutrient Regulation of Behavior" written by Professor Qingchun Tong from the University of New Texas, who specializes in research on the hypothalamus. This article reviewed the above research findings from the perspectives of nutrient sensing, functional studies, and signal regulation of AgRP neurons.
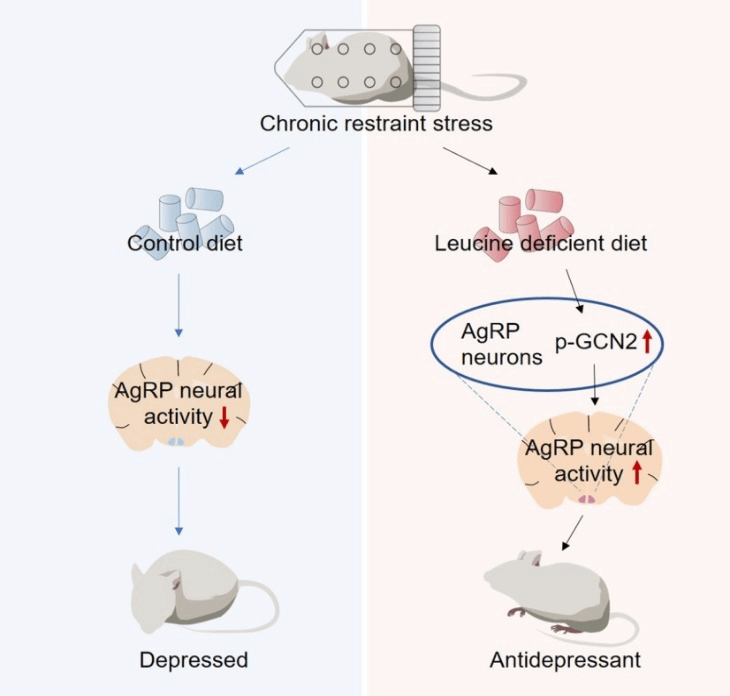
Firstly, the author examined whether short-term leucine deficiency would cause behavioral changes in non-stressed mice during the depression test. Wild-type mice were given either a control diet or a leucine-deficient diet. The depression-related behaviors of the mice were detected using open field test (OFT), elevated plus maze test (EPM), tail suspension test (TST), and forced swimming test (FST). The results showed no significant differences between the two groups of mice, indicating that short-term leucine deficiency does not affect the depression-related behaviors of non-stressed mice. The author further explored the effect of this diet on depression model mice. Using chronic restraint stress to induce depression-related behaviors, mice were fed with either a control diet or a leucine-deficient diet, and then behavioral tests were conducted. Compared with the restraint stress model mice fed with the control diet, the restraint stress model mice fed with the leucine-deficient diet showed increased center time and center distance in the open field test, increased time and entry number in the open arm of the elevated plus maze test, decreased immobility time in the tail suspension test and forced swimming test, indicating that the leucine-deficient diet alleviated the depression-like behaviors induced by chronic restraint stress. Similar effects were also observed when the other seven essential amino acids (isoleucine, valine, lysine, methionine, phenylalanine, threonine, and tryptophan) were deficient.
To explore the mechanism by which leucine deficiency improves depressive-like behaviors induced by chronic restraint stress, the authors examined the brain region closely related to emotional regulation and amino acid sensing - the hypothalamus. After the mice were subjected to a leucine deficiency diet treatment, the leucine level in the hypothalamus decreased. Injections of leucine into the third ventricle could inhibit the beneficial effect of leucine deficiency on depressive-like behaviors. At the same time, injections of leucine analog (leucine analog can increase the intracellular level of uncharged leucine-tRNA by inhibiting leucyl-tRNA synthetase, thereby simulating leucine deficiency [12]) into the third ventricle could simulate the improvement of depressive-like behaviors caused by leucine deficiency. In addition, the activity of AgRP neurons in the hypothalamus changed during chronic restraint stress and leucine deficiency. Chemical genetic inhibition of AgRP neurons eliminated the antidepressant effect of leucine deficiency. Finally, it was found that in AgRP neurons, the amino acid sensor GCN2 mediated the behavioral effects regulated by leucine deficiency. Knocking down GCN2 in AgRP neurons blocked the antidepressant effect of leucine deficiency under CRS. Chemical genetic activation of AgRP neurons reversed the effect of GCN2 knockdown.
In conclusion, this study is the first to demonstrate that the deficiency of a single essential amino acid, including leucine, has a significant alleviating effect on the depressive-like behaviors induced by chronic restraint stress in mice. These effects depend on the activity of GCN2 and AgRP neurons. The study discovered the role of AgRP neurons in amino acid sensing, provided a new dietary intervention for alleviating depressive-related behaviors, and established the important role of GCN2 in depressive-related behaviors.
Yuan Feixiang, a young associate researcher in the Institute for Translational Brain Research of Fudan University, Wu Shangming, a master's student at the Shanghai Institute of Nutrition and Health of the Chinese Academy of Sciences, and Zhou Ziheng, a doctoral student, are the co-first authors of this research. Guo Feifan, the professor in the Institute for Translational Brain Research of Fudan University, is the corresponding author of this research. This research was supported by the National Natural Science Foundation of China (31830044, 91957207, 81870592, 82270905, 81970742, 82000764, 82170868, 81970731) and the National Key Research and Development Program (2018YFA0800600).
Original link:https://doi.org/10.1093/lifemeta/load004
References
[1]. Malhi G. S. and Mann J. J. Depression. Lancet 2018; 392: 2299-2312.
[2]. Otte C., Gold S. M., Penninx B. W., et al. Major depressive disorder. Nat Rev Dis Primers 2016; 2: 16065.
[3]. Sabella D. Antidepressant Medications. Am J Nurs 2018; 118: 52-59.
[4]. Wang S. M., Han C., Bahk W. M., et al. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med J 2018; 54: 101-112.
[5]. Sarris J., Logan A. C., Akbaraly T. N., et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015; 2: 271-4.
[6]. Lang U. E., Beglinger C., Schweinfurth N., et al. Nutritional aspects of depression. Cell Physiol Biochem 2015; 37: 1029-43.
[7]. Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron. 2019;102(1):75-90.
[8]. Xia G, Han Y, Meng F, He Y, Srisai D, Farias M, Dang M, Palmiter RD, Xu Y, Wu Q. Reciprocal control of obesity and anxiety-depressive disorder via a GABA and serotonin neural circuit. Mol Psychiatry. 2021;26(7):2837-2853.
[9]. Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, Zarate CA Jr. Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol Psychiatry. 2017;81(10):886-897.
[10]. Hoepner C. T., McIntyre R. S. and Papakostas G. I. Impact of Supplementation and Nutritional Interventions on Pathogenic Processes of Mood Disorders: A Review of the Evidence. Nutrients 2021; 13.
[11]. Sparling T. M., Waid J. L., Wendt A. S., et al. Depression among women of reproductive age in rural Bangladesh is linked to food security, diets and nutrition. Public Health Nutr 2020; 23: 660-673.
[12]. Hao S., Sharp J. W., Ross-Inta C. M., et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 2005; 307: 1776-8.