Researchers report new model to study central nervous system immune cells
Microglial cells are the maintenance workers of the central nervous system (CNS), protecting against pathogens and pruning damaged neurons to help the brain maintain homeostasis. Considered immune cells, microglia work to protect the brain from before it is fully formed through its lifetime, but they aren’t infallible.The cells can be primed early on to respond in certain ways, making the microglia’s clean-up efforts less efficient. As other cells age, they can complicate microglial function, making them less effective.
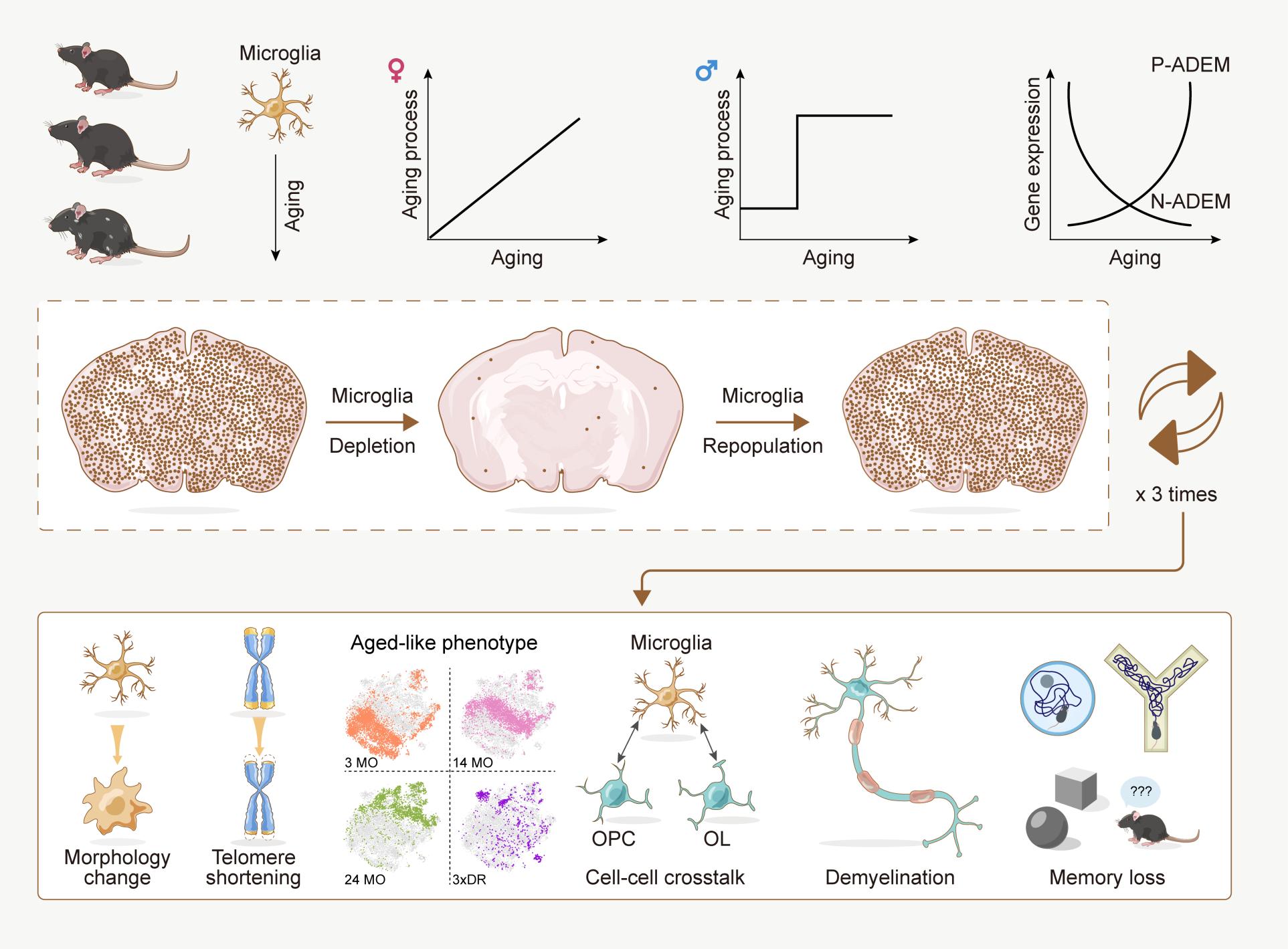
But the underlying mechanism of how microglial cells age and how their aging directly affects the brain is poorly understood — meaning that attempts to prevent or treat brain dysfunction may not be as effective as they could be, according to a multi-institutional collaboration led by Bo Peng and Yanxia Rao, both professors at Fudan University. The team investigated how microglial cells change as they age in both male and female mice across their lifespans, finding what the researchers called “unexpected sex differences.” They also established a model to study aged microglial cells in a non-aged brain, revealing that aged-like microglia contribute to cognitive decline even in young mice.
The researchers published their findings on 11/Sep/2023 in Nature Aging, entitled "Transcriptional and epigenetic decoding of the microglial aging process".
“Age is among the top risk factors to several brain disorders, and microglial cell aging contributes to the brain dysfunction,” said Peng, co-corresponding author on the paper. Peng is affiliated with the Institute for Translation Brain Research.
To better understand how microglial cell aging contributes to brain dysfunction, the team profiled microglial transcriptomes — how the microglia express their genetic directions for structure and function — at regular intervals throughout the lifespan for female and male mice via RNA sequencing, allowing the researchers to map which genes the microglia contain and which they express.
“Our results revealed that the gene profiles of microglia from the female brain gradually changed during the aging process, indicating progressive aging,” said Rao, co-corresponding author. Rao is affiliated with the Department of Laboratory Animal Science. “Unexpectedly, microglia from male mice did not show a stepwise transition during the aging process or an intermediate middle-aged stage. Instead, they displayed a young phenotype before 9 months and precipitously switched to an aged phenotype after 12 months. Female and male microglia exhibited different aging trajectories in the brain."
To better understand why female mice’s microglia display a middle age but male mice’s microglia switch suddenly from young to old, the researchers analyzed the transcriptomes at all matched ages, from 2 months to 24 months. They found that female and male microglia express hundreds of genes differently at each age, and some were positively or negatively correlated with age. Dubbed age-dependent microglia (ADEM) genes by the researchers, they identified 57 such genes in female mice and 14 in male mice.
“To precisely depict the development of ADEM genes during aging, we mapped scores of ADEM genes at single-cell resolution,” Rao said, explaining that the results confirmed that ADEM genes reliably represent core characteristics of microglial cell aging.
The researchers then analyzed ADEM genes’ transcriptional and epigenetic — genetically and environmentally induced — alterations to an immune-triggering substance. They found that microglia at different ages responded differently to the challenge, indicating that aged microglia are less responsive.
To test whether other aged cells contribute to the less stringent response, the team inhibited a receptor exclusively expressed in myeloid cells — a family to which microglia belong — to remove most of the microglial cells from the brain. Once the inhibition is removed, Peng said, the residual microglia rapidly repopulate the whole brain by proliferation. After three rounds of this process — called 3-round depletion-repopulation, or 3xDR — each microglial cell has proliferated more than 20 times and presents with an aged or aged-like phenotype, while the other brain cells remain young.
“3xDR allows researchers to decode the independent functions of aged-like microglia excluding contributions from other aged cells,” Peng said. “By using 3xDR, we revealed that aged-like microgliaper secontribute to cognitive decline and myelin impairment.”
Myelin is the material that coats nerve cells and that facilitates quick communications across fibers. Damage to myelin results in slower or stopped communications, resulting in brain dysfunction.
According to the researchers, more research is needed to understand if the microglial features observed in mice map to humans. If it does, they said, their data — available to the public atMicrogliAtlas(http://www.microgliatlas.com) — will be helpful to understand the aging process and disease mechanism in humans.
“This study aims to achieve a deep understanding of age-related changes in microglia, decipher the molecular mechanism underlying microglial cell aging and correlate microglial behaviors with brain aging,” Peng said. “Collectively, our work provides a comprehensive resource for decoding the aging process of microglia, shedding light on how microglia maintain brain function.”
In addition to Rao and Peng, the research team includes Xiaoyu Li, Yuxin Li, Yuxiao Jin, Yuheng Zhang, Jingchuan Wu, Shuai Gao, Taohui Liu, Yafei Wang, Wenxu Wang, Yousheng Shu, Feifan Guo, Wei Lu, Ying Mao from Fudan University; Zhen Xu and Yubin Huang, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences; Ti-Fei Yuan, Shanghai Jiao Tong University School of Medicine; Lin Cai and Hengli Tian, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine; and Fanzhou Zeng and Xifan Mei, The First Affiliated Hospital of Jinzhou Medical University. Cai and Zeng are also affiliated with Fudan University.
TheSTI2030-Major Projects (China Brain Project) supported by Ministry ofScience and Technology of China,National Natural Science Foundation of China; the Shuguang Program, supported by the Shanghai Education Development Foundation and Shanghai Municipal Education Commission; the Program of Shanghai Academic/Technology Research Leader; Shanghai Pilot Program for Basic Research; The Innovative Research Team of High-Level Local University in Shanghai; Shanghai Municipal Science and Technology Major Project; and the Shenzhen Science and Technology Research Program funded this research.
Paper URL: https://www.nature.com/articles/s43587-023-00479-x