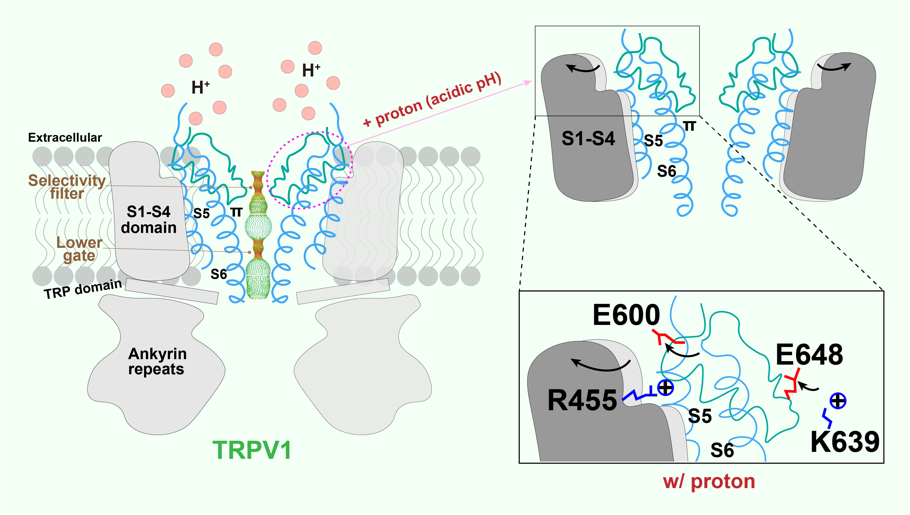
Local tissue acidosis is a driver of pain hypersensitivity, which is mediated, in part, through TRPV1. Structure-function studies have identified two negatively charged residues (E600 and E648) as key sites underlying sensitivity of TRPV1 to extracellular protons. By capturing structures over a range of pH conditions, we now map conformational transitions that reveal how sequential protonation of key Glutamic acid residues facilitates movement of elements within the outer pore region and voltage sensor-like domain that promote the channel’s open state. (For details, please refer to Zhang et al., 2021. Cell).