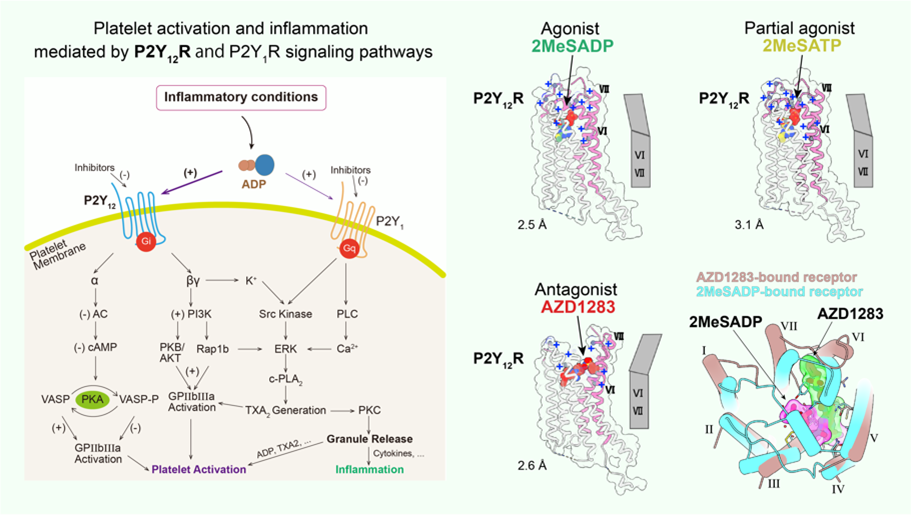
Purinergic receptor P2Y12 plays an important role in platelet activation and thrombotic disorders, which is the key target of antithrombotic drugs such as plavix, a popular inhibitor of platelet aggregation on the market. Nevertheless these drugs have side effects including indigestion and excessive bleeding etc. P2Y12R pathway is not only responsible for platelet activation, but also involved in inflammatory response and functional regulation of glial cells which has been increasingly recognized by experimental evidences. To better understand the molecular mechanism of P2Y12R ligand recognition and facilitate drug development targeting P2Y12R, we determined the high-resolution three-dimensional structures of P2Y12R bound to antagonist AZD1283, full agonist 2MeSADP and partial agonist 2MeSATP, respectively. These results, combined with computer docking experiments, suggest that residue C97, not forming a disulfide bridge, belongs to a covalent binding pocket for the active metabolites of P2Y12R drugs. These observations are consistent with the absolute selectivity of the active metabolites. The hypothetical binding modes of P2Y12R enhance our understanding of the ligand recognition of purinergic receptors and the function of GPCR superfamily. Although both 2MeSADP and AZD1283 bind to the same pocket, their orientations are completely different, with only partial overlap between them, in contrast with the basically overlapping orientations previously observed for other receptors. Surprisingly, as a first example of a GPCR, there are striking conformational changes between nucleotide and nonnucleotide ligand complexes in the extracellular regions, as the agonist-bound pocket is sealed by a ‘lid’ formed by unusually cationic ECLs and the N terminus. These findings provide useful insights into GPCR activation and the diversity of the GPCR-mediated cell signaling. (For details, please refer to Zhang et al., 2014a. Nature; Zhang et al., 2014b. Nature).