Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) is a progressive neurological disease with an average age of onset of 43 years and an average life expectancy of only 3 to 5 years after symptoms begin. ALSP is caused by microglial mutation, the immune cells of the central nervous system (CNS). Currently, ALSP has no cure and treatments are limited.
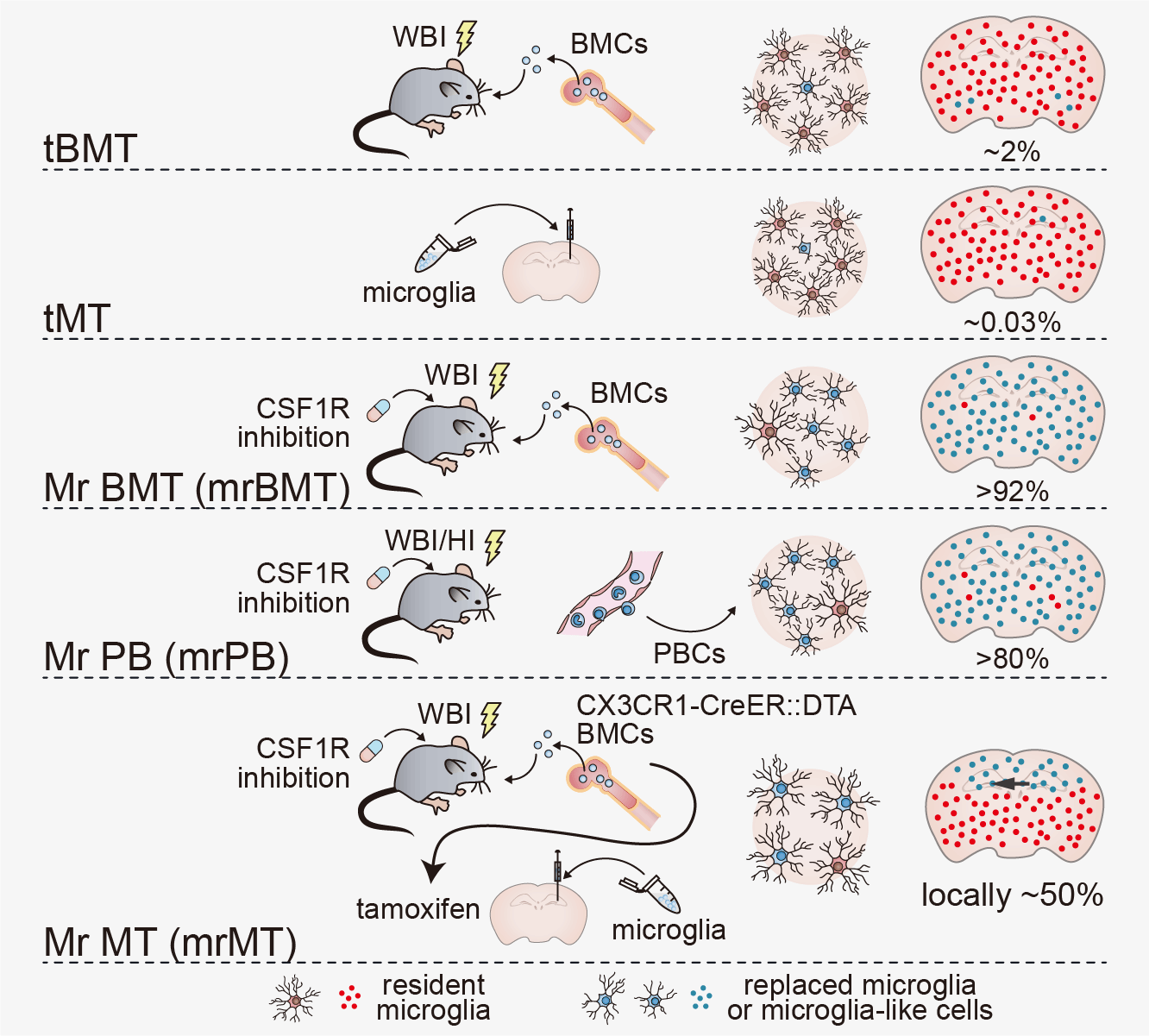
All microglia rely on a kinase called colony-stimulating factor 1 receptor (CSF1R), which is only found in microglia and other myeloid cells. When CSF1R gene carries pathogenic mutations, brain microglia are affected and causes the fatal disease ALSP. Thus, the microglial CSF1R gene is identified as a possible target for ALSP treatment. Bo Peng, a professor at Fudan University in Shanghai, China, first developed efficient strategies for microglia replacement in 2020. These treatment methods for treating diseases were currently named "microglia intervention strategy for therapy and enhancement by replacement," or MISTER. Prof. Peng and his colleagues utilized one of the microglia replacement strategies, Mr BMT (microglia replacement by bone marrow transplantation, aka mrBMT), replace CSF1R-expressing microglia with wild-type CSF1R-expressing microglia. The exciting results of microglia replacement in mouse models and clinical treatments in humans with ALSP were published in Science on 10/Jul/2025.
"ALSP is a fatal disease with no current curative treatment. Since pathogenic mutations in microglia-specific gene CSF1R are the cause of ALSP, we reasoned that replacing CSF1R-deficient microglia with wild-type microglia would halt disease progression. Our findings demonstrate that microglia replacement effectively corrects the pathogenic CSF1R mutations in both mice and human individuals with ALSP, halting disease progression and improving neurological function," said Bo Peng.
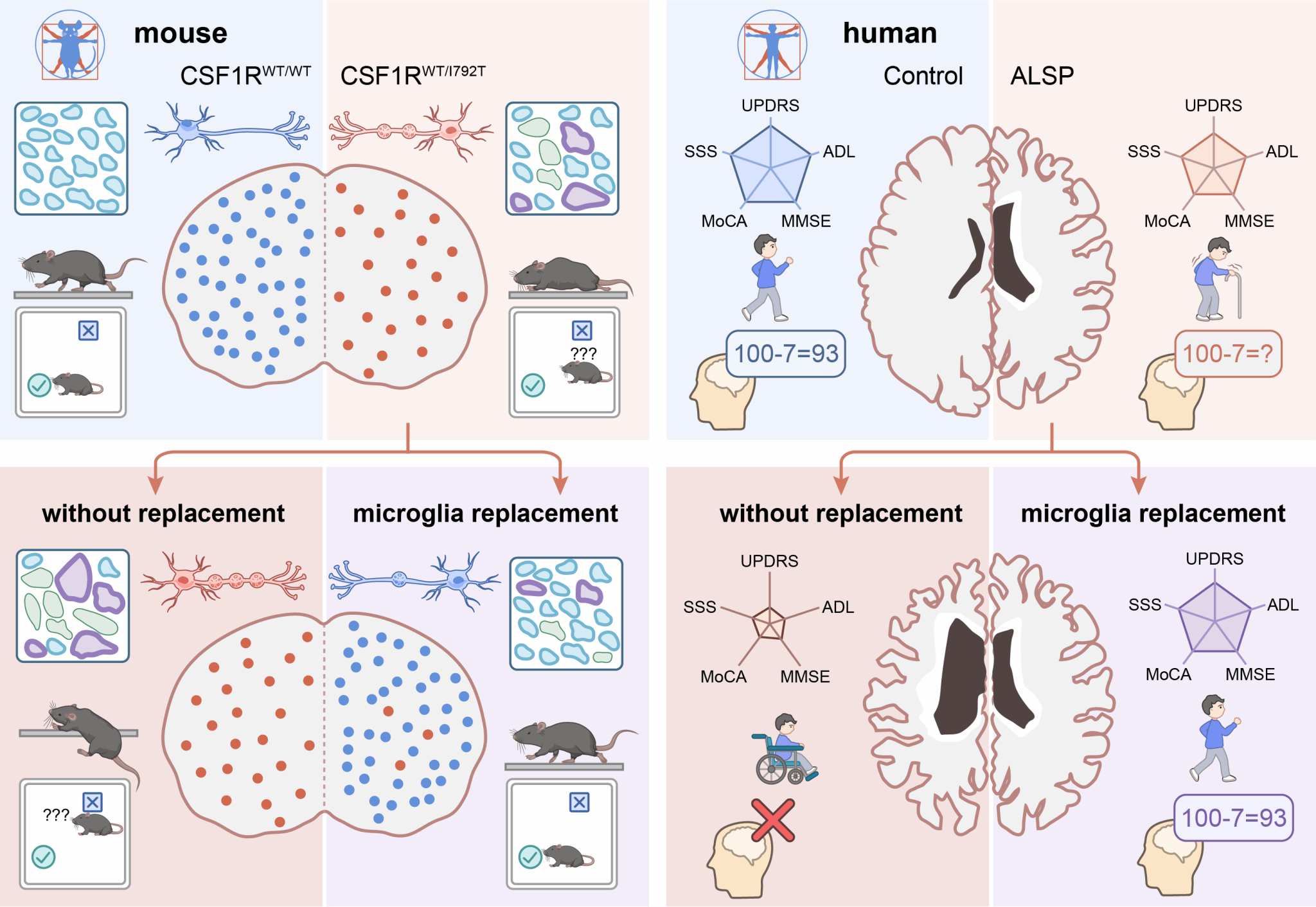
Because ALSP is so rare, the disease has lacked adequate animal models and there has been limited understanding of how ALSP develops. So researchers first developed animal models based on human hotspot mutations, which reflect the pathological symptoms and progression of ALSP in humans. Mice with these mutations had significantly reduced microglia, along with hallmark physical signs of ALSP such as myeline pathology, axonal swelling and spheroids in the brain, plus motor impairments and cognitive symptoms. After the similarities between the mouse models and human progression of ALSP were confirmed, researchers replaced mutant CSF1R-expressing microglia with CSF1R-normal microglia through Mr BMT. Researchers found that 91.15% of the microglia were replaced after Mr BMT and the amount of microglia in the ALSP mice increased. Myeline pathology, axonal swelling and spheroids were rescued as well. Behavioral tests showed that Mr BMT significantly improves motor and cognitive performances for ALSP mice.
After the success of the mouse models, researchers studied the treatment in humans through a clinical trial. The procedure of Mr BMT is pharmacological CSF1R inhibition followed by bone marrow transplantation. Because people with ALSP per se have CSF1R mutations, CSF1R inhibition is not necessary and bone marrow transplantation is sufficient for microglia replacement, which were demonstrated by their mouse experiments. There were eight people with ALSP who received a bone marrow transplantation-based microglia replacement from CSF1R-normal donors. Magnetic resonance imaging (MRI) was used to observe ALSP disease progression in these patients. Those who did not receive microglia replacement had severe atrophy and other signs of disease progression in the following 12 months. In comparison, those patients who underwent microglia replacement had no disease progression for 24 months after transplantation. The microglia replacement also stabilized cognitive and motor function for at least 24 months.
"For the first time, we have achieved microglia replacement in animal models and shown promising results in the human clinical trial. This is currently the only effective clinical treatment for ALSP. Microglia replacement, which was developed in our lab in 2020, has therapeutic potential beyond ALSP for other neural diseases, too," said Peng.
Looking ahead, researchers hope to apply the strategies learned during this study to other neural diseases. "We previously developed efficient strategies for microglia replacement, opening up a new cell therapy strategy that has therapeutic potential for treating neural diseases. Now, we demonstrated the efficacy by this first microglia replacement for clinical therapy with very good therapeutic results. Microglia replacement is the only effective clinical treatment for ALSP. We hope to utilize microglia replacement to conquer more diseases," said Peng.
Jingying Wu, Yafei Wang, Xiaoyu Li, Pei Ouyang are the co-first authors, Li Cao and Yanxia Rao are the co-corresponding authors, and Bo Peng is the last corresponding author of this study. Other contributors include Yuanyuan Cai, Yang He, Mengyuan Zhang, Xinghua Luan, Yuxiao Jin, Jie Wang, Yujie Xiao, Yuqing Liang, Fang Xie, Yousheng Shu, Jiong Hu, Chunkang Chang, Jieling Jiang, Dong Wu, Youshan Zhao, Taohui Liu, Yuxin Li, Xiaojun Huang, Yao Li, Junfang Zhang, Yuwen Cao, Xin Cheng and Ying Mao.
STI2030-Major Projects, National Natural Science Foundation of China, Shanghai Pilot Program for Basic Research, Program of Shanghai Academic/Technology Research Leader, The Innovative Research Team of High-Level Local University in Shanghai, Program for Outstanding Medical Academic Leader of Shanghai, Shanghai Science and Technology Innovation Action Plan, the Training Program for Research Physicians of Innovative Translational Ability Supported by Shanghai Hospital Development Center, and the Program of Brain Science and Brain-Like Intelligence Technology by Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine supported this research.
paper link: https://doi.org/10.1126/science.adr1015