Autism spectrum disorder (ASD), affecting approximately 1% of the global population, significantly impacts the patients' ability to communicate, learn, and adapt to daily life. Core symptoms include impairments in social interaction and repetitive behaviors. Hundreds of autism-associated risk genes have been identified, many of which are involved in synaptic protein synthesis, chromatin remodeling, and transcriptional regulation. A distinct class of risk genes, such as SCN2A, stands out for its role in regulating neuronal excitability and enabling action potential generation.
SCN2A encodes the α-subunit of voltage-gated Na+ channel NaV1.2, which is one of the most abundantly expressed Na+ channel isoforms in the brain. Loss-of-function mutations in SCN2A represent a strong risk factor for ASD, but the precise pathological mechanisms remain unclear. To dissect the underlying mechanisms, previous studies focused on the function of Scn2a in the neocortex and revealed that a complete loss of Scn2a increases the excitability of neocortical pyramidal neurons, while heterozygous loss exerts no effect on the firing frequency. Moreover, Scn2a deletion in the prefrontal cortex failed to produce social deficits in mice.
The current study from Dr. Yousheng Shu's research team at the Institute for Translational Brain Research, Fudan University, focuses on the ventral tegmental area (VTA), a region critical for processing social reward. Based on their earlier findings that dopaminergic neurons (DANs) in the VTA highly express NaV1.2 in the axon initial segments, Dr. Shu's team hypothesized that Scn2a dysfunction may contribute to pathogenesis of ASD by impairing the function of DANs. This work is published online by Neuron on July 10, 2025, with the title: Selective loss of Scn2a in ventral tegmental area dopaminergic neurons leads to dopamine system hypofunction and autistic-like behaviors.
Dr. Shu's team first carried out immunofluorescence staining combined with in situ hybridization, and found that Scn2a is the most abundant Na+ channel isoform in VTA DANs. Conditional deletion of Scn2a in these neurons significantly reduced their excitability. Notably, around 17% of dopaminergic neurons failed to fire action potentials, indicating a cell-type heterogeneity.
Then, they performed dual patch-clamp recordings from the soma and axon, showing that NaV1.2 is essential for action potential conduction along DAN axons. Patch-clamp recording from the soma, axon trunk, and terminal branches in the nucleus accumbens (NAc) revealed significant contribution of NaV1.2 to Na+ currents along the somato-axonal axis. Imaging experiments in brain slices using dopamine fluorescence sensors showed a dramatic reduction in dopamine release at NAc following Scn2a deletion.
Notably, mice with Scn2a deletion in VTA DANs displayed autistic-like behaviors, including hyperactivity, repetitive circling, reduced anxiety, decreased sociability, and a lack of social novelty. During a task to distinguish a stranger mouse from a familiar one, these mice exhibited decreased levels and insufficient dynamics of dopamine release in the NAc when interacting with social partners, suggesting a key role of dopamine system hypofunction in mediating the impaired sociability.
Inspired by the use of levodopa for treating the Parkinson's disease, the team found that a moderate dose of levodopa significantly enhances both baseline and evoked dopamine release in Scn2a-deficient mice. Surprisingly, levodopa produces no obvious effect on motor behaviors (e.g., hyperactivity) but greatly alleviates the non-motor deficits, as reflected by the rescue of the insufficient anxiety and the impaired sociability.
Importantly, these findings were replicated in a more clinically relevant model, Scn2a heterozygous mice. These mice exhibited similar autistic-like behaviors, including hyperactivity, reduced anxiety, impaired sociability, and deficits in social novelty preference and recognition. Importantly, the levodopa treatment significantly improved their non-motor behavior deficits.
This study uncovers a crucial role of NaV1.2 in regulating action potential generation and dopamine release in DANs. For the first time, the research team revealed that Scn2a deletion in VTA DANs alone induces repetitive behaviors and social deficits, and levodopa effectively relieves the core non-motor autistic-like symptoms in both conditional knockout mice and heterozygous Scn2a mice (Figure 1). These results establish dopamine system hypofunction as a key driver of SCN2A-associated ASD, and provide a potential pharmacological treatment for ASD individuals with SCN2A loss-of-function mutations or even others with dopamine hypofunction.
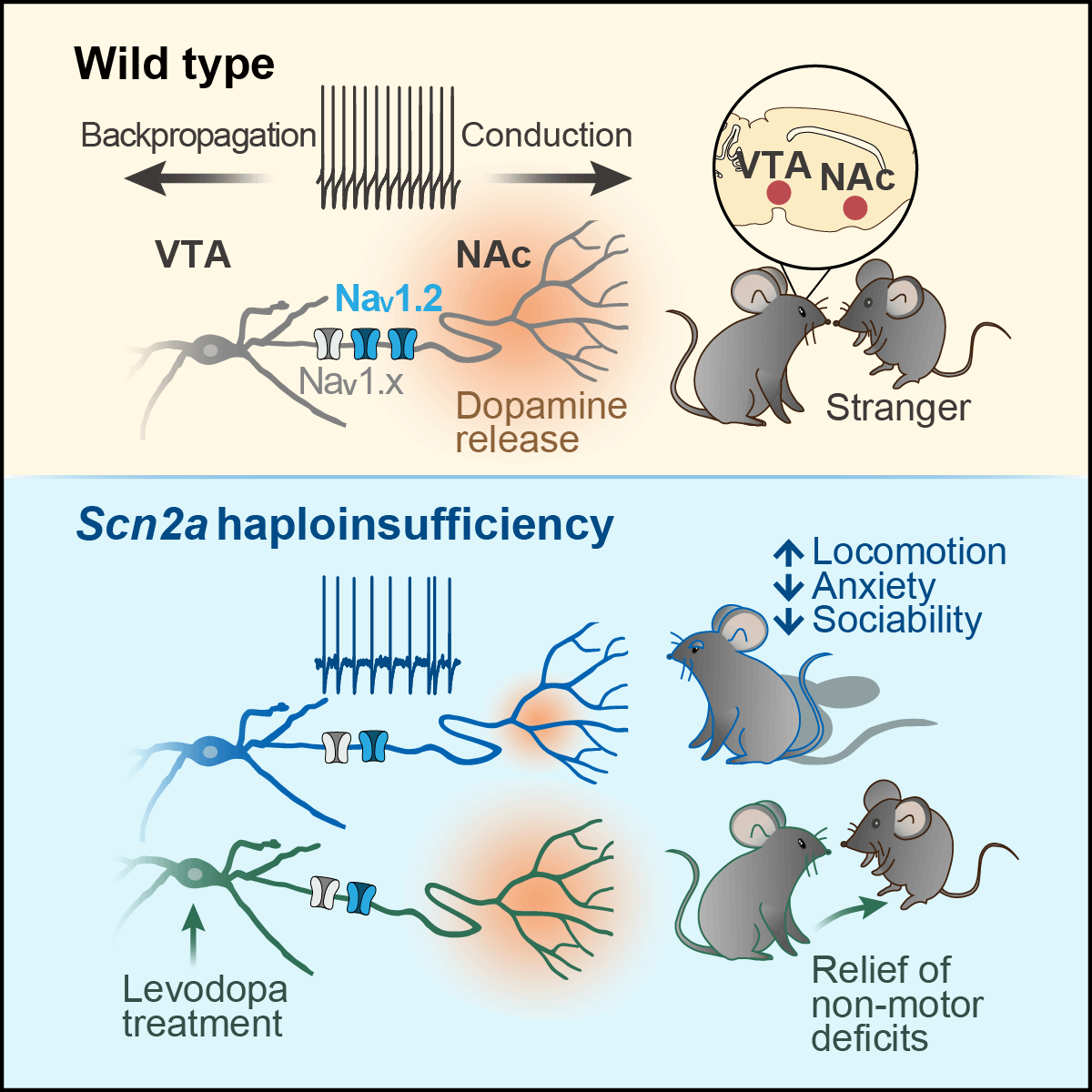
Figure 1. Scn2a deletion in DANs reduces neuronal excitability and dopamine release, leading to autistic-like behaviors. Importantly, levodopa treatment can rescue the non-motor behavior deficits.
This work was led by Prof. Yousheng Shu at the Institute for Translational Brain Research, Fudan University. PhD students Liang Li and Qi Huang are co-first authors. Significant contributions were also made by PhD student Jiahao Hu (Bo Li's lab), Dr. Mengmeng Jin (Jie He's lab), and Dr. Yizhou Zhuo (Yulong Li's lab).
The study was supported by the National Natural Science Foundation of China (32130044 and T2241002), Ministry of Science and Technology of China (STI2030-Major Projects 2021ZD0202500), and Program of Shanghai Academic/Technology Research Leader (21XD1400100).
Paper URL: https://doi.org/10.1016/j.neuron.2025.06.003
https://www.cell.com/neuron/abstract/S0896-6273(25)00434-9