On April On April 30, 2025, the research group of Li Baobin from the Institute for Translational Brain Research at Fudan University and the research group of Wang Jin from the School of Science at China Pharmaceutical University published a research paper titled "Gating mechanism of the two-pore-domain potassium channel THIK1" in the journal Nature Structural & Molecular Biology. The paper reports the high-resolution cryo-EM structure of the two-pore potassium channel THIK1 and its mutant. Additionally, the study combines whole-cell electrophysiological experiments and molecular dynamics simulations to reveal the unique gating mechanism of THIK1, laying the foundation for developing regulators of THIK1.
The two-pore potassium channel THIK1 mainly localizes to the surface of microglia and regulates its physiological functions, such as immune surveillance, morphological branching, and phagocytic clearance. Dysfunction of THIK1 is closely associated with the development of autism, multiple sclerosis, and Alzheimer's disease. Therefore, THIK1 is a key therapeutic target for neurological disorders. The activity of THIK1 is affected by various factors, including temperature, polyunsaturated fatty acids (PUFAs), isoflurane, halothane, and G proteins. Understanding how THIK1 opens and closes is crucial for clarifying its physiological role and discovering new regulators.
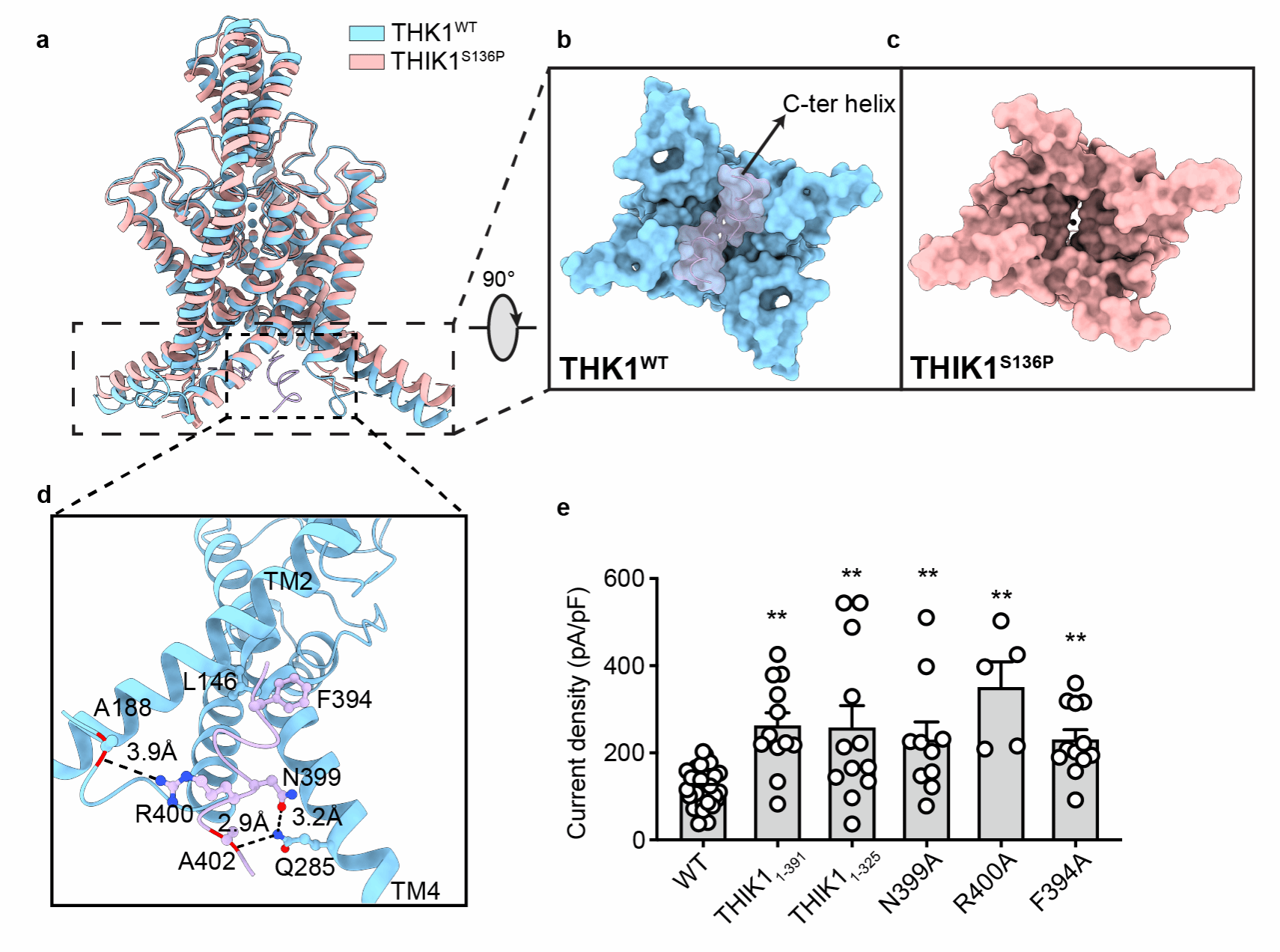
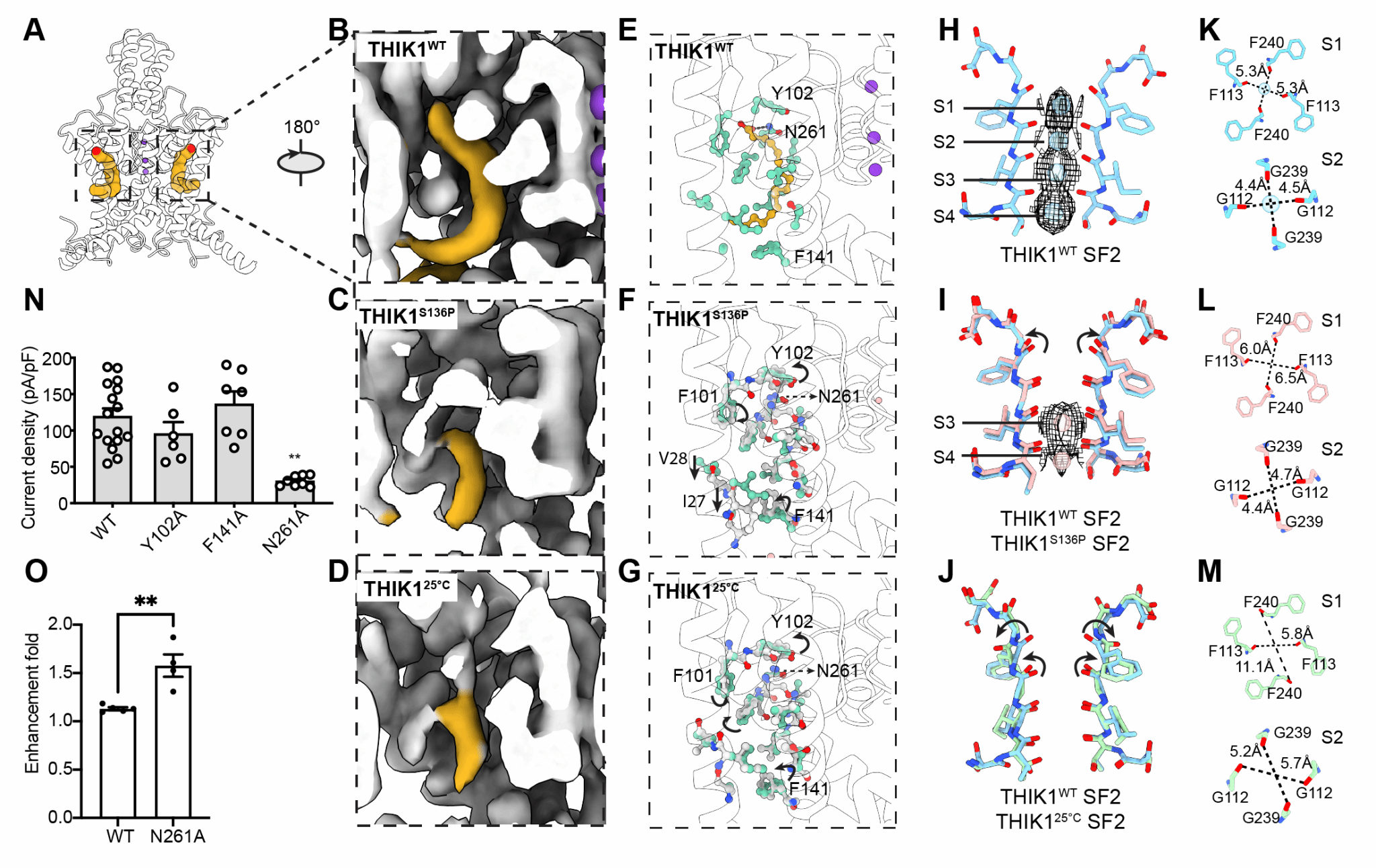
This study determined the cryo-EM structures of full-length wild-type (WT) human THIK1 (THIK1WT) and S136P (THIK1S136P) mutant at overall resolutions of 2.7 Å and 3.1 Å. THIK1 exhibits typical K2P structural features, consisting of two subunits. Each subunit contains four transmembrane helices (TM) and two pore-forming motifs that form the potassium ion selectivity filter (SF) of K2P. Notably, THIK1 shows a series of structural features and gating mechanisms that differ from other K2Ps. Two inner gates within THIK1 regulate potassium ion transport. The first inner gate is located below the selectivity filter and is formed by a pair of tyrosine residues (Tyr273). The benzene rings of tyrosine create steric hindrance, restricting the potassium conduction pathway below the SF. Below the intracellular vestibule of THIK1, a pair of short C-terminal helices form inner gate II, blocking the entrance of the intracellular vestibule and hindering potassium conduction. Structural and electrophysiological experiments on THIK1S136P demonstrated that the C terminus can directly respond to various physiological stimuli, possibly involving caspase 8 cleavage and G protein regulation.
In addition, the study also found that there is a hydrophobic pocket of about 20 Å near SF1 of the two subunits, which is composed of TM2 and TM4 of one subunit and TM1 of the adjacent subunit. A pair of polyunsaturated fatty acids (PUFA) occupies this hydrophobic pocket, which can stabilize the selectivity filter and regulate channel activity.
Fang Xiangyun, a doctoral student at the Institute for Translational Brain Research at Fudan University, and Jin Haichao, a master's student at the School of Science at China Pharmaceutical University, are the co-first authors of the paper. Li Baobin, Principal Investigator at the Institute for Translational Brain Research at Fudan University, Zhang Ran, Junior Co-Principal Investigator at the same institute, and Wang Jin, Principal Investigator at the School of Science, China Pharmaceutical University, are the co-corresponding authors. This research was funded by the Science and Technology Innovation 2030 - "Brain Science and Brain-like Research" Major Project (Youth), the National Natural Science Foundation of China, and other foundation projects.