In recent years, the global incidence of obesity has risen significantly, posing a major public health threat. Consequently, a deep understanding of the relationship between obesity and energy balance has become a central focus in metabolic research. Adipose tissue is no longer viewed merely as a passive energy storage depot but is recognized as a crucial metabolic hub, playing a key role in energy expenditure. Thermogenesis, a vital form of energy expenditure, actively consumes excess energy to maintain systemic energy homeostasis. Brown adipose tissue (BAT), rich in mitochondria, acts as a core effector in energy metabolism regulation by dissipating substantial energy through non-shivering thermogenesis.
Concurrently, nutrient sensing mechanisms play a pivotal role in the precise regulation of energy homeostasis. Intracellular nutrient sensing pathways dynamically monitor changes in nutrient concentrations (e.g., glucose, amino acids) and regulate energy metabolism through related signaling cascades. The brain, acting as the "central command center" for metabolic regulation, has been a major research focus regarding how it senses nutritional status and modulates energy balance. While amino acid (e.g., leucine) deficiency has been shown to improve metabolism, the underlying neural sensing mechanisms and regulatory circuits remained elusive.
On May 1, 2025, Professor Guo Feifan's team at the Institute for Translational Brain Research, Fudan University, published a research paper titled "Amino acid-sensing Neurons in the Anterior Piriform Cortex Control Brown Adipose Tissue Thermogenesis" in the international journal Advanced Science. This study, for the first time, identified corticotropin-releasing hormone (CRH) neurons in the anterior piriform cortex (APC) as "leucine sensors" whose activity is regulated via the amino acid sensing signal GCN2. The team further delineated the APCCRH-LH amino acid-sensing neural circuit that regulates BAT thermogenesis to improve metabolism, providing a novel perspective for obesity treatment.
Traditionally, amino acid sensing was thought to primarily involve the brainstem and hypothalamus. This new study, however, focused on the APC, a region associated with olfaction but rarely linked to metabolic regulation. The team hypothesized that specialized "amino acid-sensing neurons" might exist in the APC, remotely controlling adipose tissue metabolism via neural circuits.
Using C57BL/6J mice, the researchers conducted a series of experiments. Feeding mice a leucine-deficient diet for 3 days increased their rectal temperature. Infrared thermography revealed elevated surface temperature over BAT, along with reduced lipid droplets and increased expression of key thermogenic components like uncoupling protein 1 (UCP1), indicating enhanced BAT thermogenesis. Immunofluorescence staining showed that leucine deficiency increased the number of c-Fos-positive neurons in the APC, predominantly excitatory glutamatergic neurons. Chemogenetic experiments further confirmed that inhibiting APC glutamatergic neurons blocked leucine deficiency-induced BAT thermogenesis.
Further investigation revealed that CRH neurons within the APC are sensitive to leucine deficiency, which activates them. Chemogenetic inhibition of APCCRH neurons significantly reduced the thermogenic effects induced by leucine deprivation, while activating these neurons mimicked thermogenic activation, demonstrating their key regulatory role in BAT thermogenesis. Leucine deficiency lowered leucine levels in the APC. Real-time calcium imaging captured neuronal responses to leucine deficiency, showing significantly increased firing activity in approximately 22% of APCCRH neurons. Brain slice patch-clamp recordings determined that this response depended on the GCN2 pathway. Knocking down GCN2 blocked leucine deficiency-induced BAT thermogenesis, confirming GCN2's critical role.
Regarding neural circuitry, anterograde and retrograde tracing techniques revealed that APCCRH neurons project to the lateral hypothalamus (LH). Inhibiting the APC-to-LH projection weakened leucine deficiency-induced BAT thermogenesis, whereas activating this projection promoted BAT thermogenesis, establishing the APC-LH circuit as essential for regulating BAT thermogenesis.
Additionally, the study found that APCCRH neurons act as "cold-defense guards." During cold exposure (4 ℃), these neurons were activated. Mice with chemogenetically activated APCCRH neurons maintained stable body temperature, while those with inhibited neurons exhibited significantly greater heat loss. This suggests these neurons integrate environmental temperature signals to dynamically regulate thermogenic efficiency, functioning as a "dual-regulator" for metabolism and survival.
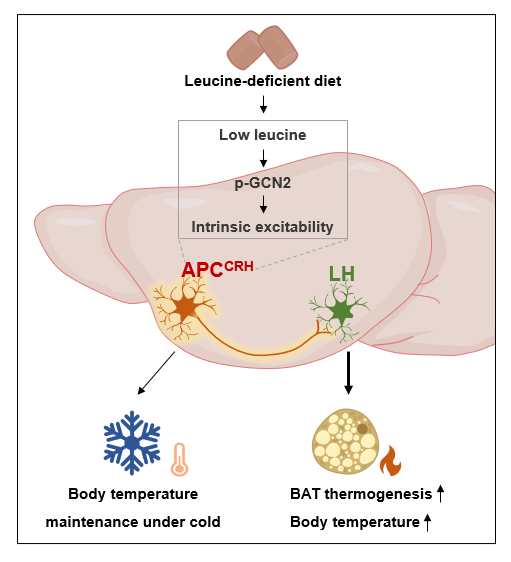
In summary, this study is the first to identify leucine-sensing CRH neurons in the APC. These neurons modulate their excitability via the GCN2 pathway and regulate BAT thermogenesis, body temperature, and fat metabolism through the APC-LH neural circuit. This work not only expands our understanding of amino acid-sensing neuron functions but also provides novel potential therapeutic targets for chronic metabolic diseases like obesity and type 2 diabetes. It also offers new perspectives for managing metabolic diseases in cold environments (e.g., preventing winter cardiovascular and cerebrovascular events), potentially paving new therapeutic avenues.
Professor Guo Feifan and Associate Research Scientist Yuan Feixiang from the Institute for Translational Brain Research, Fudan University, are the co-corresponding authors. Peixiang Luo, a Ph.D. student at the Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, is the first author. This research was supported by grants from the National Natural Science Foundation of China and the National Key R&D Program of China.