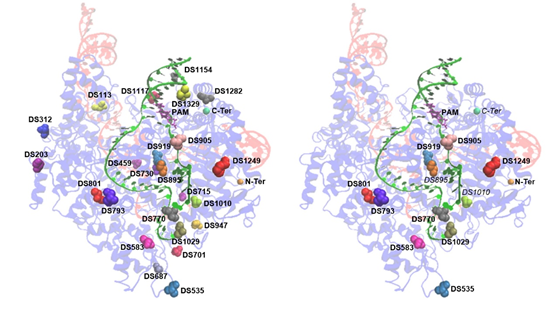
Conventionally, ABEs were constructed by fusing adenosine deaminases to either the amino terminus (N-terminus) of Cas9. In this study, internal fusion strategy is deployed to improve ABE tools. When fusing with adenosine deaminases, 11/24 docking sites inside Cas9 could result in functional ABE variants (ABE-nSpCas9-DSs). New functional ABE variants could be classified into two major groups depending on their editing scopes: one is similar to traditional ABEs, with editing scope in A4-A7 (the PAM was counted as 21–23) and the other displays obviously shifted editing scopes in A9-A16, which has not been reported previously. Our study diversifies the editing windows and provides novel insights into the optimization of ABE tools. Taken together, this study could serve as a guide for safety improvement and application expansions for base editing tools. (Nature Communications,2020,doi:10.1038/s41467-020-19730-9)