On July 8, 2022, a research group led by Prof. Wei-Guang Li from our institute, in collaboration with Prof. Tian-Le Xu’s lab at the Basic Medical College of Shanghai Jiao Tong University and Prof. Fan Jiang’s team at the National Children’s Medical Center (Shanghai)/Shanghai Children's Medical Center, published a landmark paper entitled “Dynamic tripartite construct of interregional engram circuits underlies forgetting of extinction memory” in the international journal Molecular Psychiatry. By integrating sophisticated techniques such as behavioral assays, activity-dependent engram labeling, optogenetics, viral neural circuit tracing, electrophysiological recordings, and in vivo fiber photometry, the authors systematically dissected the neural mechanisms underlying extinction memory—a newly formed inhibitory memory during fear extinction training. This pioneering study revealed the fundamental principles governing extinction memory formation, storage, retrieval, and eventual forgetting, thus providing novel insights into relapse phenomena frequently observed following treatment for severe emotional disorders such as depression, anxiety, and post-traumatic stress disorder (PTSD).
Traumatic experiences can generate intense, enduring emotional memories, known as pathological emotional memories. These memories are easily formed yet notoriously resistant to forgetting, persistently intruding into daily life and significantly contributing to psychiatric conditions like depression, anxiety, and PTSD. Fear memory vividly illustrates this process, often consolidating robustly in the brain. Extinction training—an evolutionarily conserved adaptive mechanism—counteracts such entrenched fear by generating a new, inhibitory extinction memory. Clinically, extinction-based exposure therapy is widely used and highly effective in correcting cognitive biases and alleviating symptoms of emotional disorders. Unfortunately, extinction memories are notably fragile, often rapidly deteriorating or becoming ineffective, leading to the spontaneous resurgence or relapse of the original fear memory. Hence, elucidating the precise neural substrates underlying extinction memory, along with its dynamic patterns of storage and retrieval, is essential for understanding emotional regulation and preventing the recurrent relapses commonly associated with affective disorders.
Contemporary neuroscience postulates that memories are stored in distinct neuronal ensembles called engram cells, whose reactivation is essential for memory recall. Increasing evidence indicates that memories are not encoded solely by stable, hard-wired neural connections between distinct brain regions but rather through dynamic interactions among distributed engram-cell populations. Using targeted recombination in active populations (TRAP) technology, the researchers demonstrated that extinction memories are not localized to isolated brain regions but are dispersed across multiple interconnected cortical and thalamic areas.
Focusing specifically on extinction memory engram cells within the medial prefrontal cortex (mPFC), basolateral amygdala (BLA), and ventral hippocampus (vHPC), the authors revealed that optogenetic silencing or activation of these distinct cellular ensembles respectively inhibited or enhanced extinction-memory retrieval. Subsequent functional validations combined with rabies-virus-based circuit tracing unexpectedly identified a distinctly unidirectional connectivity pattern among these engram populations. Rather than reciprocal or bidirectional projections, synaptic connections predominantly extended from BLA to mPFC and from vHPC to mPFC, orchestrating the dynamic retrieval of extinction memories.
Utilizing optogenetic manipulations, ex vivo electrophysiological slice recordings, and in vivo fiber photometry, the team further discovered that spontaneous recovery of extinguished fear was accompanied by weakening of these specialized engram-to-engram synapses. Crucially, renewed extinction training or artificial induction of synaptic long-term potentiation (LTP) via optogenetics successfully reversed the weakening of these synapses and suppressed fear relapse. Collectively, these results demonstrate that synaptic connections linking extinction memory engram cells distributed across multiple brain regions form an integrated engram network, whose dynamic remodeling directly controls extinction memory retrieval and maintenance (see schematic figure in the original paper).
By characterizing the directional connectivity and dynamic plasticity of an mPFC-centered extinction-memory engram network, this study provides the most direct neurobiological evidence yet explaining the inherent fragility and vulnerability of extinction memories. These findings lay a robust theoretical foundation for developing anti-relapse interventions in emotional disorders, offering promising new therapeutic directions. Furthermore, this work provides a valuable conceptual framework and experimental paradigm applicable to understanding the neural principles of other memory types, significantly advancing our comprehension of emotional information encoding within the brain.
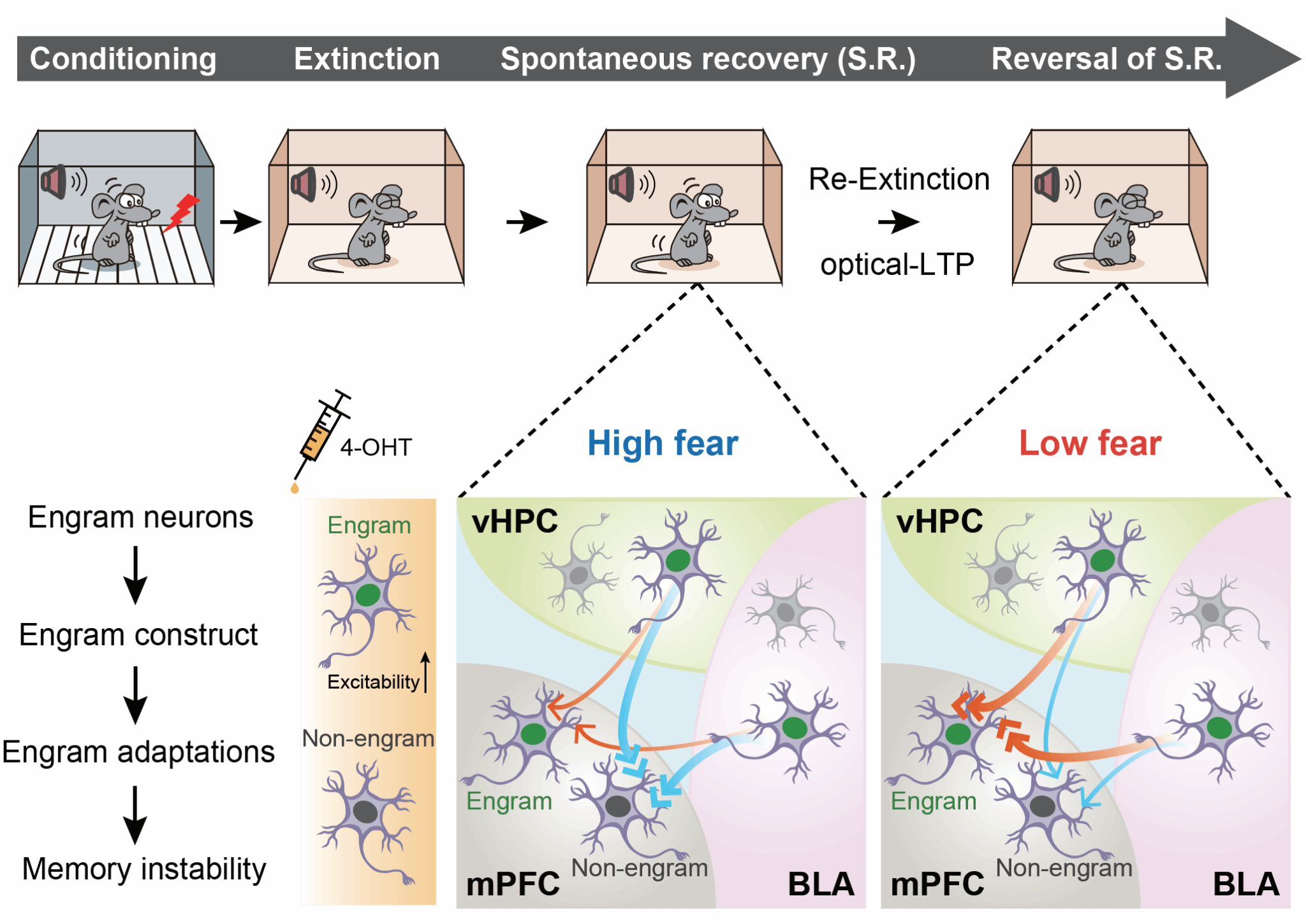
Figure. Schematic model illustrating the mechanism by which a dynamic engram network mediates extinction memory from its formation to forgetting.
Authorship and Acknowledgments:
Graduate student Xue Gu and postdoctoral researcher Dr. Yan-Jiao Wu from Shanghai Jiao Tong University are co-first authors of this paper. Profs. Wei-Guang Li, Tian-Le Xu, and Fan Jiang are co-corresponding authors.
This work was generously supported by multiple funding sources, including the “Brain Science and Brain-Inspired Intelligence” Major Project (Science & Technology Innovation 2030), the National Natural Science Foundation of China, Shanghai Municipal Major Science & Technology Projects, Key Programs of the Shanghai Science and Technology Commission, Innovation Teams supported by Shanghai Municipal Education Commission, and the Original Exploration Youth Program of the Frontier Research Center for Basic Medicine at Shanghai Jiao Tong University. The authors also acknowledge the collaborative contributions from Prof. Lu-Yang Wang (University of Toronto), Prof. Michael X. Zhu (UTHealth, Houston), and Prof. Nan-Jie Xu (Shanghai Jiao Tong University).
Link to original paper: https://doi.org/10.1038/s41380-022-01684-7