On September 11, 2024, the research team led by Professor Guo Feifan at our institute published a study titled "Hypothalamic SLC7A14 accounts for aging-reduced lipolysis in white adipose tissue of male mice" in the international academic journal Nature Communications.
Using an aging mouse model combined with genetic, metabolomic, and single-cell sequencing analyses, the study revealed the role and mechanism of the hypothalamic amino acid transporter SLC7A14 in aging-induced white adipose tissue lipolysis through the brain-gut-fat axis. The findings uncover a novel function of central SLC7A14, providing new insights into the central regulation of age-related lipolysis impairment and identifying a promising drug target for treating age-dependent obesity.

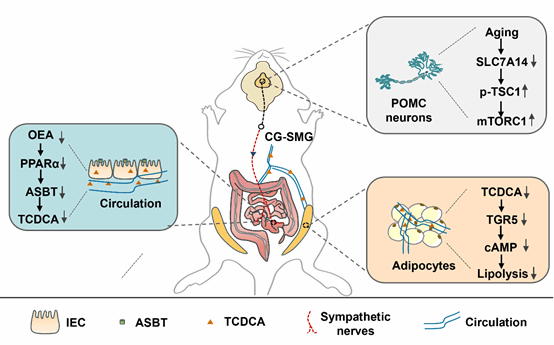
China is currently undergoing significant demographic changes, marked by a declining proportion of youth and a notably increasing proportion of middle-aged and elderly populations. Consequently, preventing and managing obesity in middle-aged and elderly individuals has become an urgent health priority for the future. However, the pathogenic mechanisms underlying age-dependent obesity remain largely unclear. In elderly individuals, increased fat accumulation is often accompanied by reduced lipolysis in white adipose tissue. Studies have reported that the central nervous system, particularly the hypothalamus, plays a crucial role in regulating white adipose tissue lipolysis. Moreover, mTOR signaling in hypothalamic POMC neurons is essential for maintaining body weight in aging mice. Nevertheless, the role and mechanism of mTOR signaling in age-induced reduction of lipolysis remain poorly understood. SLC7A14, an amino acid transporter located on lysosomal membranes, is highly expressed in the central nervous system. However, its functional role within the CNS remains unclear.
In this study, the authors employed an aging mouse model and discovered that SLC7A14 expression was significantly reduced in the hypothalamic arcuate nucleus of aged mice. Overexpression of SLC7A14 in POMC neurons ameliorated age-related lipolysis impairment in white adipose tissue (WAT) of aged mice.Subsequently, the authors investigated the mechanism by which SLC7A14 in POMC neurons regulates lipolysis dysfunction in aged mice. While it is well-established that the central nervous system primarily regulates lipolysis through sympathetic innervation of adipose tissue, the authors unexpectedly found that SLC7A14 in POMC neurons does not mediate its effects through this pathway after blocking sympathetic nerves in WAT.Interestingly, through metabolomic profiling and mass spectrometry analysis, the authors revealed that SLC7A14 in POMC neurons modulates serum levels of the primary bile acid taurochenodeoxycholic acid (TCDCA). They further demonstrated that TCDCA, acting through its receptor TGR5, mediates the lipolysis impairment induced by either SLC7A14 knockout or aging. Additional investigations showed that SLC7A14-regulated TCDCA levels depend on the intestinal bile acid transporter ASBT.The study further uncovered that POMC SLC7A14 modulates ASBT expression and TCDCA levels by regulating the OEA-PPARα axis through sympathetic nerves projecting to the intestine. Finally, the authors demonstrated that POMC SLC7A14 suppresses mTOR signaling by inhibiting TSC1 phosphorylation, thereby regulating the activity of intestinally-projecting sympathetic nerves.This research elucidates the molecular mechanisms underlying central regulation of age-dependent obesity and provides novel insights into how the brain-gut-adipose axis mediates aging-induced lipolysis dysfunction.
Dr. Jiang Xiaoxue (postdoctoral fellow at the Institute for Translational Brain Research, Fudan University) and Mr. Liu Kan (Ph.D. candidate at the Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences) are co-first authors of this paper. Prof. Guo Feifan (Institute for Translational Brain Research, Fudan University/Zhongshan Hospital) serves as the corresponding author. This research was supported by grants from the National Natural Science Foundation of China and the National Key Research and Development Program of China.