Following the groundbreaking publication by Peng Bo's team in *Nature Neuroscience* on February 22, 2018, which successfully unraveled the mystery of the origin of repopulated microglia in the brain (Huang et al., 2018), the journal *Cell Discovery*, published by the Nature Publishing Group, featured another series of papers by the same research team on February 27, 2018, focusing on the origin of repopulated microglia.
In their study titled "Dual extra-retinal origins of microglia in the model of retinal microglia repopulation" (Peng et al., 2018), Peng Bo's team reported for the first time the phenomenon of microglial repopulation in the retina and discovered a completely different dual extra-retinal origin pathway compared to brain microglial repopulation. This is the first time that extra-retinal origins and migration pathways of microglia have been identified in the adult mammalian retina, and it is also the first report of large-scale cellular regeneration in the adult mammalian retina. The study holds significant scientific value for understanding the maintenance of microglial homeostasis and the mechanisms of retinal degenerative diseases.
Background: Origins of Repopulated Microglia in the Brain
The regenerative capacity of the adult mammalian central nervous system (CNS), including the brain, retina, and spinal cord, is extremely limited. For a long time, it was believed that damage to the CNS could not be repaired.
In 2014, researchers from the University of California, Irvine, discovered an astonishing phenomenon of cellular regeneration in the mouse brain. By specifically inhibiting the colony-stimulating factor 1 receptor (CSF1R), they were able to almost completely eliminate microglia in the brain (>99%) (Elmore et al., 2014). When the inhibition was stopped and a brief recovery period ensued, new microglia appeared in the brain. These repopulated microglia rapidly colonized the entire brain and fully restored to their original state (such as density, distribution, and morphology) within about a week (Elmore et al., 2014). This was the first time that large-scale cellular regeneration had been observed in the adult mammalian CNS, a process termed microglial repopulation. The research team speculated through histological immunostaining that the repopulated microglia might be derived from a type of cells in the brain that transiently express Nestin. This was considered the third type of stem cells discovered in the adult mammalian CNS, in addition to neural stem cells and oligodendrocyte precursor cells. This conclusion received widespread attention upon publication but also sparked significant controversy.
On February 22, 2018, in the top neuroscience journal *Nature Neuroscience*, Peng Bo's team published a study titled "Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion" (Huang et al., 2018). Using rigorous fate mapping and lineage tracing techniques, the team systematically elucidated the origin of repopulated microglia. They overturned the previous conclusion that repopulated microglia originated from Nestin-positive precursor cells and discovered that repopulated microglia entirely originated from the proliferation of residual microglia in the brain (<1%) (Figure 3) (Huang et al., 2018). This phenomenon is the fastest cell proliferation observed in the adult mammalian CNS to date.
Surprising Discoveries in the Retina: Two New Extra-Retinal Origin Pathways
The retina is a unique part of the CNS. In adult mammals, there is no definitive evidence of the existence of stem cells with differentiation potential in the retina (only sporadic reports of cellular regeneration exist, and these are still controversial). Therefore, the retina was considered to have no regenerative capacity, and once damaged, it could not be repaired.
When Peng Bo's team used the drug PLX5622 to specifically inhibit CSF1R, they found that, unlike brain microglia, all microglia in the retina were completely eliminated (100%). If retinal microglia followed the same repopulation pathway as brain microglia, then due to the absence of residual microglia in the retina, the researchers hypothesized that microglial repopulation would not occur in the retina.
However, to their great surprise, Peng Bo's team discovered that microglia in the retina could still repopulate after the inhibition by PLX5622 was stopped. This indicated that retinal microglia have a completely different repopulation mechanism from brain microglia. Additionally, this was the first time that large-scale cellular regeneration had been observed in the adult mouse retina.
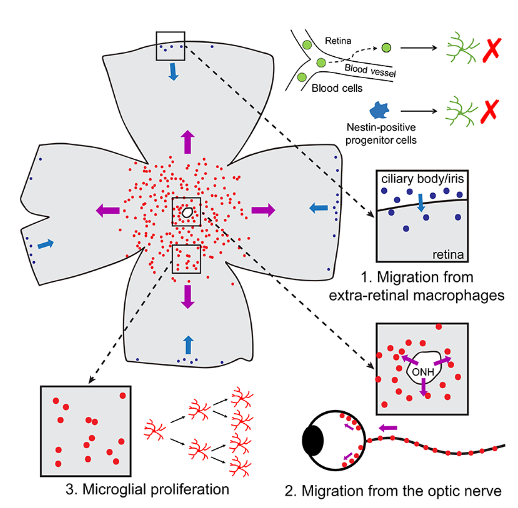
To explore the origin of repopulated microglia in the retina, Peng Bo's team used a series of lineage tracing techniques and ex vivo retinal cultures. They found that, unlike brain microglia, repopulated microglia in the retina have two distinct extra-retinal origin pathways: the majority of repopulated microglia originate from residual microglia in the optic nerve that migrate outward (Pathway 2), while a smaller portion of repopulated microglia come from macrophages in the ciliary body and/or iris outside the retina that migrate inward (Pathway 1). The two different origins of repopulated microglia exhibit distinct cellular morphologies, suggesting differences in their intrinsic properties and functions. Moreover, the exogenously migrated microglia retain the ability to proliferate within the retina (Pathway 3).
In summary, using the microglial depletion-repopulation model, Peng Bo's team observed large-scale cellular regeneration in the adult mammalian retina for the first time and elucidated its cellular origin. For a long time, microglia in the retina were believed to be maintained primarily by endogenous microglia, both in homeostatic and disease states. This study is the first to observe extra-retinal origins of microglia in the adult mammalian retina, a finding of significant scientific value for understanding the pathogenesis and therapeutic strategies of retinal degenerative diseases. Additionally, through their series of work on microglial repopulation in the brain and retina, the team has comprehensively elucidated the origins and functions of microglia in the CNS.
This work was primarily a collaboration between Peng Bo's team and Dr. Rao Yanxia from the University of Hong Kong, who are the co-corresponding authors. Dr. Huang Yubin and Xu Zhen from Peng Bo's team are the co-first authors of the series of studies. The work was mainly supported by the National Key R&D Program of China (2017YFC0111202), the National Natural Science Foundation of China (31600839), the Shenzhen Knowledge Innovation Program (JCYJ20170307171222692), the Guangdong Innovative Team (2013S046), and the Shenzhen Peacock Team.
Related article:
Huang Y., Xu Z., Xiong S., Qin G., Sun F., Yang J, Yuan T.F. Zhao L, Wang K, Liang Y.X., Fu L., Wu T, Lu Z, So K.F., Rao Y.* andPeng B.*(2018) Dual origins of retinal microglia in the model of microglia repopulation.Cell Discovery4, 9.
Link to related reports:
https://mp.weixin.qq.com/s/yuF0RsXj0dc5-68v59gcoQ