On December 17, 2023, Prof. Wei-Guang Li from our institute, in collaboration with Prof. Yiyun Chen's team from the Shanghai Institute of Organic Chemistry (CAS) and Prof. Tian-Le Xu's group from Shanghai Jiao Tong University School of Medicine, published a research article titled "Genetically Encoded Photocatalysis Enables Spatially Restricted Optochemical Modulation of Neurons in Live Mice" in the prestigious American Chemical Society flagship journal ACS Central Science. This study successfully developed a genetically encoded photocatalytic platform for precise neuronal modulation via spatially specific, bioorthogonal photocatalytic boronate uncaging reactions. Using this approach, the researchers achieved subcellular-specific, neural projection-specific, and cell type-specific optochemical control of neurons in living cells, isolated neurons, and live mice. Specifically, through bioorthogonal photocatalytic release of the GABA(B) receptor agonist baclofen in vivo, the team identified NaV1.8-positive neurons as the key peripheral neuronal subtype responsible for GABAergic modulation of itch sensation.
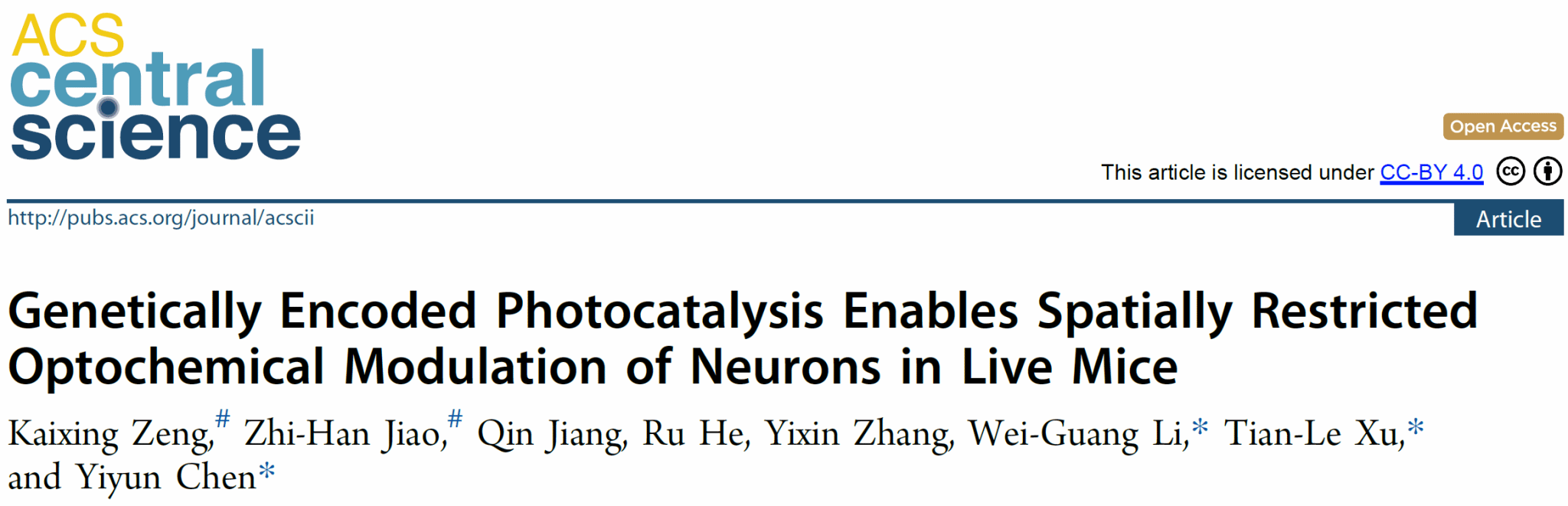
Light offers remarkable spatiotemporal precision for neuronal control, while small molecules—with their structural diversity—can target various proteins, providing substantial advantages in neuronal modulation. However, current photocaged small molecules diffuse freely after photorelease, generating uniform concentrations within illuminated areas and thus lacking the spatial specificity achieved by genetically encoded photosensitive proteins (e.g., optogenetics). In contrast, genetically encoded photocatalytic reactions produce and release bioactive molecules specifically near the photocatalyst via the "proximity effect", thereby achieving superior spatial specificity in modulating protein targets. Existing genetically encoded photocatalysis strategies primarily utilize antibody-labeled heavy-metal photocatalysts, whose neurotoxicity limits applicability in delicate neural tissues.
Prof. Yiyun Chen's laboratory at the Shanghai Institute of Organic Chemistry (CAS) has long been dedicated to developing visible-light-driven catalytic reactions based on organoboron compounds. Previously, in collaboration with Prof. Tian-Le Xu's group at Shanghai Jiao Tong University School of Medicine, they successfully employed organic fluorescent dyes—typically used in bioimaging—as biocompatible photocatalysts capable of catalyzing boronate oxidation under visible light irradiation. Through a photocatalytic redox reaction utilizing cellular oxygen and antioxidants to produce hydrogen peroxide, this method achieved deboronative hydroxylation, thus photoreleasing bioactive molecules such as baclofen with high spatiotemporal precision on mouse brain slices (Angew Chem Int Ed, 2019). In this current study, the collaborative team further developed a genetically encodable, biocompatible visible-light photocatalytic system. Leveraging genetic methods to define specific cell types or cellular compartments (Fig. 1), they aimed to realize spatially specific photocatalytic neuronal modulation.
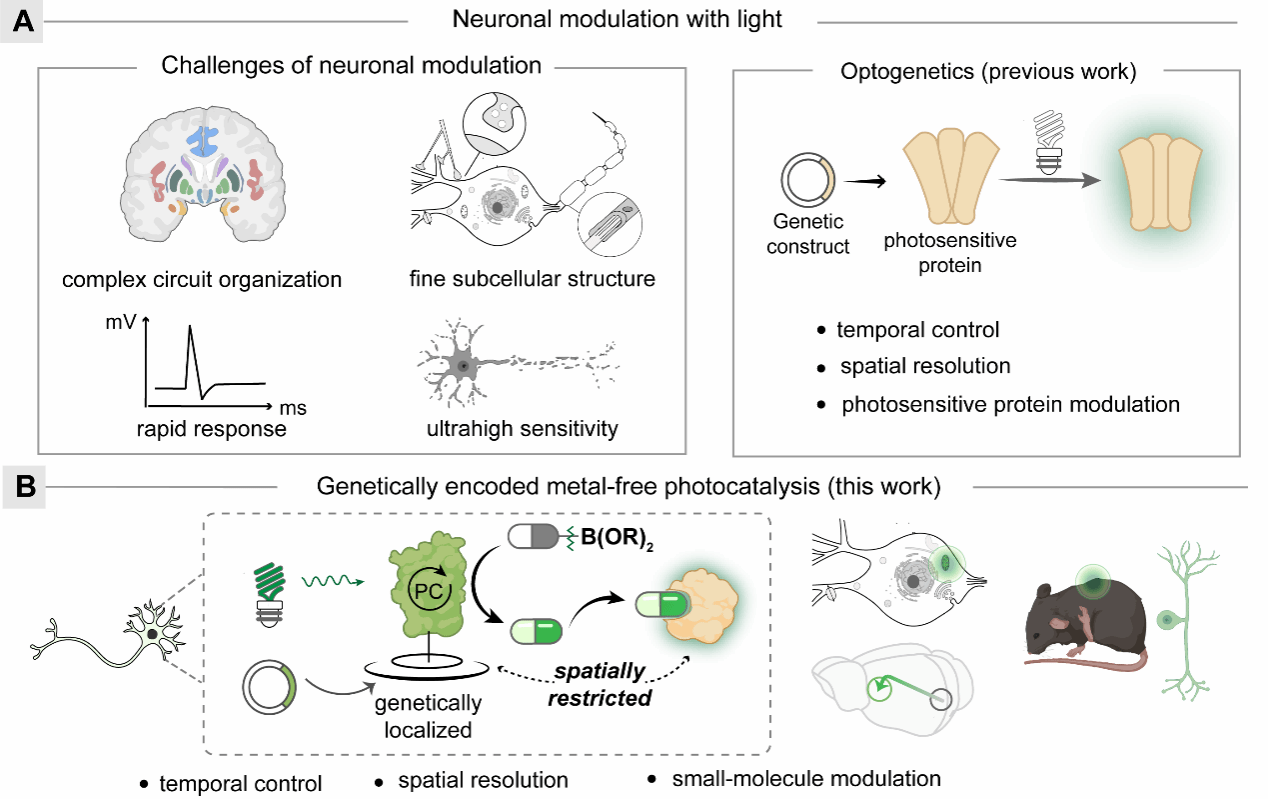
Figure 1. Optical neuromodulation and genetically encoded photocatalytic small-molecule modulation.
Building upon their previous work, the team designed and synthesized a bifunctional ligand-organic dye conjugate named BGFL, which selectively couples to SNAP-tag proteins expressed in live cells, forming a genetically encoded hybrid protein photocatalyst termed SNAP-FL. This protein-based photocatalyst exhibited excellent photocatalytic properties and photostability, efficiently and rapidly generating superoxide radical anions to promote deboronative hydroxylation and subsequent bioactive small molecule release both in vitro and in vivo (Fig. 2A). Given its genetic encodability, this method enabled spatially precise photocatalytic release of fluorescent molecules and the anticancer drug doxorubicin (DOX) in various subcellular compartments, such as mitochondria, nucleus, endoplasmic reticulum, and cytoplasm, thus achieving targeted subcellular protein function modulation (Fig. 2B). Additionally, targeted mitochondrial modulation of membrane potential was successfully demonstrated in cultured neurons via photocatalytic release of the mitochondrial uncoupler DNP (Fig. 2C).
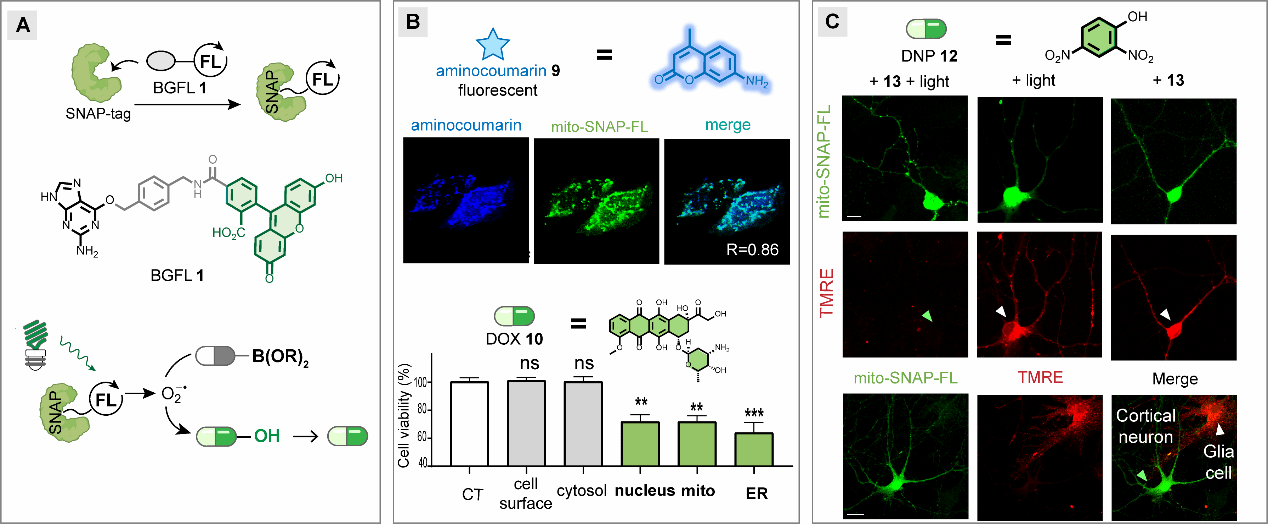
Figure 2. Genetically encoded photocatalytic small-molecule modulation of diverse cellular and neuronal functions.
The researchers further applied this technique to achieve precise modulation of long-range synaptic projections. SNAP-tag proteins were expressed specifically in cortical neurons projecting to the amygdala. After conjugation with the bifunctional ligand-organic dye BGFL to form SNAP-FL, the hybrid protein photocatalyst enabled simultaneous optochemical modulation and optogenetic/electrophysiological measurement of synaptic transmission within these projections in mice. They found that photocatalytically released baclofen exhibited projection-specific activity, selectively activating presynaptic GABA(B) receptors at defined cortical-amygdala synapses without affecting other regions. This integrated approach not only employs optogenetic methods to anchor stimulation to particular neural projections but also achieves localized, precise small-molecule release through genetically encoded photocatalytic reactions, providing neuroscientists with a versatile and powerful experimental tool.
Figure 3. Specific effects of genetically encoded photocatalytic small-molecule release technology on long-range neural projection synapses.
In live mouse experiments, researchers demonstrated, for the first time, effective itch relief via green light-driven photocatalytic release of baclofen onto mouse skin. Comparing targeted release specifically at NaV1.8-positive sensory nerve terminals with broad, pan-peripheral neuronal release, they observed that only selective photocatalytic release at NaV1.8-positive terminals effectively reduced scratching behaviors. These results identify NaV1.8-positive neurons as a critical peripheral neuronal subtype in GABA(B)-mediated itch modulation (Fig. 4).
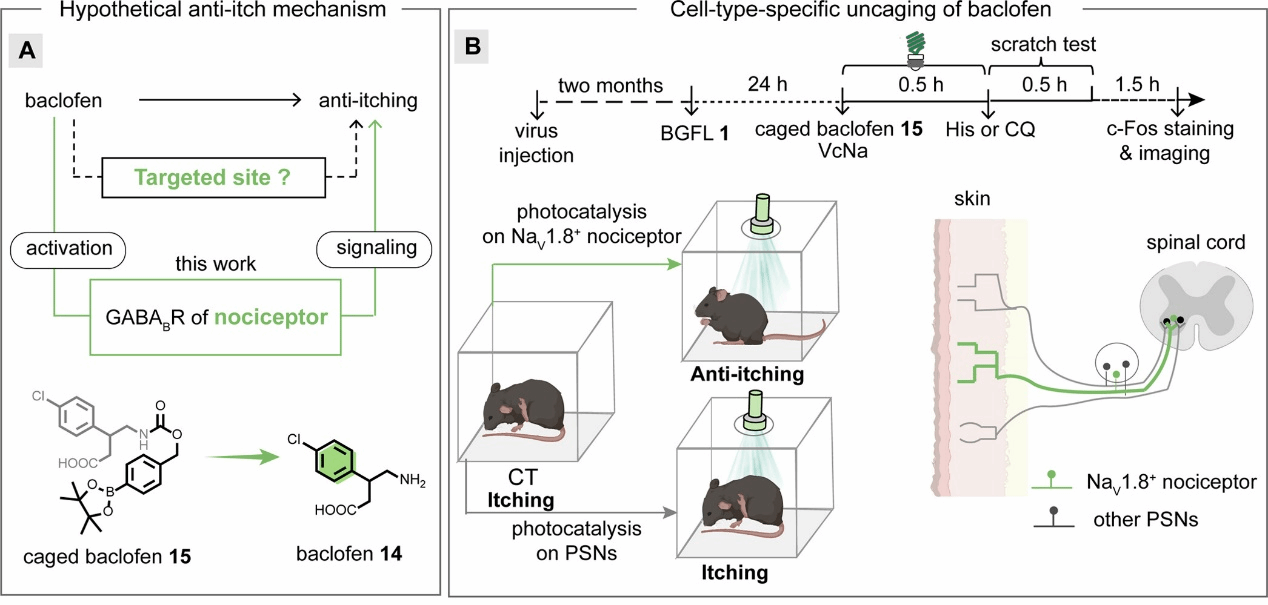
Figure 4. Dissecting sensory transmission pathways in vivo using genetically encoded photocatalytic small-molecule release technology.
Overall, this genetically encoded visible-light photocatalysis technology provides a novel avenue for real-time optochemical control of molecularly defined neuronal types and their specific compartments. It addresses multiple challenges inherent to traditional metal-based photocatalysis, such as neurotoxicity and insufficient antibody targeting efficiency, significantly enriching the neuroscientist's toolkit for optochemical applications. This breakthrough research was published in ACS Central Science, with its conceptual figure selected as the journal's front cover image.
Authorship and Acknowledgments:
Mr. Kaixing Zeng, a doctoral student jointly trained by the Shanghai Institute of Organic Chemistry (CAS) and ShanghaiTech University, and Ms. Zhi-Han Jiao, a doctoral student from Shanghai Jiao Tong University School of Medicine, are co-first authors. Prof. Yiyun Chen (Shanghai Institute of Organic Chemistry, CAS), Prof. Tian-Le Xu (Shanghai Jiao Tong University School of Medicine), and Prof. Wei-Guang Li from our institute are co-corresponding authors. This study was supported by funding from the National Natural Science Foundation of China, the Chinese Academy of Sciences Interdisciplinary Innovation Team Project, the Shanghai Science and Technology Commission, Shanghai Jiao Tong University School of Medicine, and the State Key Laboratory of Chemical Biology, among others.
Link to original paper: https://doi.org/10.1021/acscentsci.3c01351