On June 14, 2024, a collaborative research team led by Prof. Wei-Guang Li from our institute, in conjunction with teams headed by Chief Physician Mazhong Zhang from the Children's Brain Science Center at the National Children's Medical Center (Shanghai), Department of Anesthesiology and Clinical Pharmacology Lab at Shanghai Children's Medical Center affiliated with Shanghai Jiao Tong University School of Medicine, and Director Yan Luo from Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine, published a study entitled "An ultra-short-acting benzodiazepine in thalamic nucleus reuniens undermines fear extinction via intermediation of hippocamposeptal circuits" in the journal Communications Biology. Using the rapid-onset and ultra-short-acting benzodiazepine anesthetic remimazolam as a model, this study systematically elucidated how benzodiazepines affect fear extinction through specific neural circuitry. The research demonstrates that benzodiazepines, commonly used as anxiolytics, while effectively alleviating anxiety symptoms, also interfere significantly with fear extinction. These findings provide critical new insights into resolving the clinical conflict arising from combining benzodiazepine-based anti-anxiety treatments with fear-extinction-based exposure therapies in anxiety disorders and post-traumatic stress disorder (PTSD).
Anxiety disorders constitute a prevalent mental health challenge in contemporary society, with benzodiazepines frequently prescribed for symptom relief. Fear extinction, a form of inhibitory learning and memory that helps organisms adapt to environmental changes, serves as the theoretical basis for behavioral and cognitive therapies targeting anxiety disorders and PTSD. Clinical and preclinical studies have suggested that benzodiazepines, when combined with extinction-based exposure therapy, may negatively impact fear extinction and safety-signal recognition. Specifically, benzodiazepine exposure during extinction training creates drug-induced interoceptive states that differ from the drug-free state during retrieval tests, triggering renewal of extinguished fear memories through context shifts. Moreover, benzodiazepines may disrupt fear extinction by reducing conditioned fear responses and thereby impairing prediction-error-driven new learning. However, previous attempts to delineate benzodiazepine effects on fear extinction have often been confounded by sedative, hypnotic, or anesthetic side effects. Thus, utilizing an ultra-short-acting benzodiazepine may clarify its precise impact on fear extinction while minimizing interference from drug-induced internal states.
Remimazolam, a novel benzodiazepine anesthetic with rapid onset, ultra-short duration, and swift recovery profile, was employed in this collaborative study led by Dr. Li and colleagues to investigate benzodiazepine effects on fear extinction and their underlying neural circuits. Fear extinction involves dynamic interactions within a tripartite neural circuit comprising the ventral hippocampus (vHPC), medial prefrontal cortex (mPFC), and basolateral amygdala (BLA). Additionally, the thalamic nucleus reuniens (RE)—with dense reciprocal projections connecting mPFC and vHPC—plays a pivotal role in coordinating these areas during fear extinction.
Researchers initially identified a specific dose range of remimazolam that impairs fear extinction without affecting locomotor activity. Whole-brain immunohistochemical staining of the immediate-early gene c-fos indicated significant remimazolam-induced decreases in neuronal activity within RE and vHPC, accompanied by increased activity in the lateral septum (LS), suggesting a reconfiguration of neuronal activity across fear extinction-related brain regions.
Targeted infusion of remimazolam into RE recapitulated systemic administration, similarly impairing fear extinction. Electrophysiological experiments revealed that remimazolam, as a benzodiazepine, potentiates GABAergic synaptic transmission via enhanced GABA(A) receptor activity, consequently reducing neuronal excitability in RE. Genetic knockdown of the GABA(A) receptor γ2 subunit specifically in RE neurons abolished remimazolam's effects on fear extinction. Circuit-specific genetic rescue experiments further demonstrated that remimazolam's impairment of fear extinction specifically requires RE neurons projecting to vHPC (RE-vHPC neurons). Additional experiments showed remimazolam impairs fear extinction by enhancing long-range GABAergic transmission from LS to RE. Consistently, optogenetic activation of LS-to-RE projections mimicked remimazolam's impairment of fear extinction.
Finally, by delineating neural connections among RE, vHPC, and LS, the researchers identified a positive-feedback neural circuit that promotes fear extinction (Fig. 1): RE sends monosynaptic excitatory projections to vHPC, vHPC, in turn, sends feedforward inhibitory projections onto LS GABAergic neurons, and LS GABAergic neurons subsequently project back onto RE, forming a positive-feedback loop. Remimazolam selectively disrupts this circuit at the level of RE, weakening the fear extinction process.
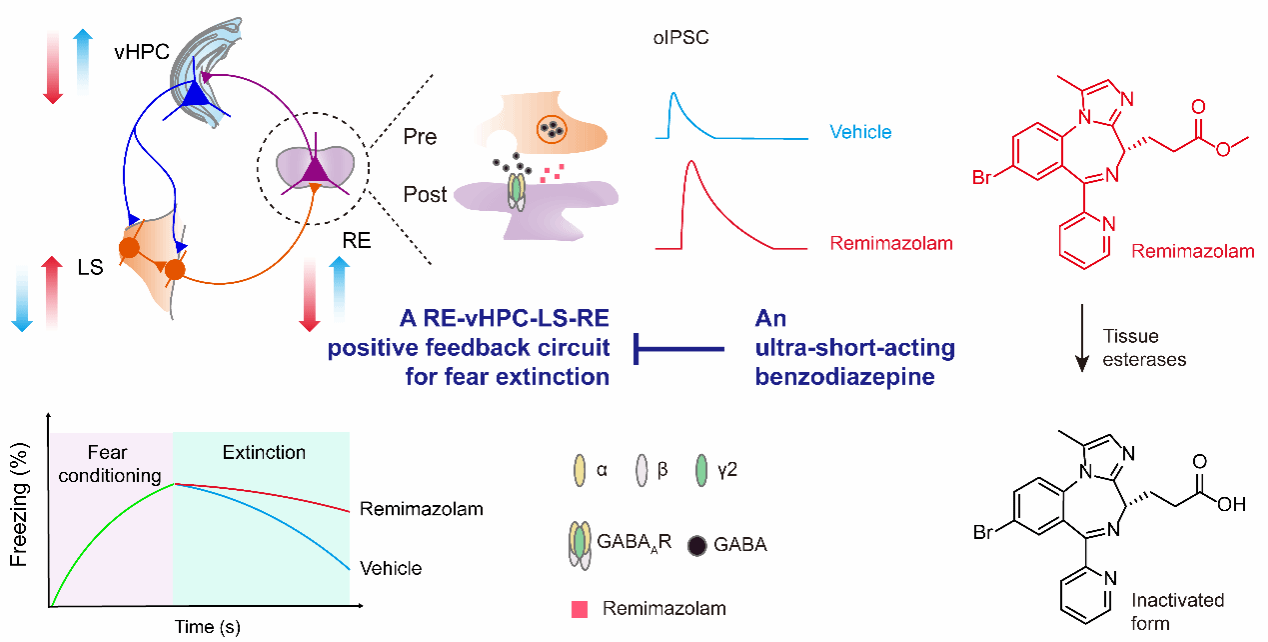
Figure 1. Neural mechanism by which remimazolam impairs fear extinction via modulation of the RE→vHPC→LS→RE circuit.
In conclusion, this study not only clarifies the specific neural circuit mechanisms through which benzodiazepines impair fear extinction but also provides an important theoretical foundation for clinical strategies combining benzodiazepine treatments with extinction-based exposure therapies. These findings offer novel insights and potential advancements in the clinical treatment of anxiety disorders and PTSD.
Authorship and Acknowledgments:
Dr. Hoiyin Cheung (doctoral graduate from the Department of Anesthesiology, Shanghai Children's Medical Center affiliated with Shanghai Jiao Tong University School of Medicine and the Children's Brain Science Center of National Children's Medical Center, Shanghai), Master's student Mr. Tong-Zhou Yu, and postdoctoral fellow Dr. Xin Yi from our institute contributed equally as co-first authors. Prof. Wei-Guang Li from our institute, Prof. Mazhong Zhang (Deputy Dean of Shanghai Children's Medical Center, Director of the Department of Anesthesiology, and President of Shanghai Children's Medical Center Guizhou Hospital), and Director Yan Luo (Department of Anesthesiology, Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine) serve as co-corresponding authors.
This research received funding from the Science and Technology Innovation 2030 Major Project ("Brain Science and Brain-Inspired Research"), the National Natural Science Foundation of China, and various grants from Shanghai municipal funding agencies. Collaborative support was provided by Prof. Tian-Le Xu from Shanghai Jiao Tong University School of Medicine and Director Jijian Zheng from the Department of Anesthesiology, Shanghai Children's Medical Center affiliated with Shanghai Jiao Tong University School of Medicine.
Link to original paper: https://www.nature.com/articles/s42003-024-06417-w