Post-traumatic stress disorder (PTSD) is a debilitating psychiatric condition characterized by delayed yet persistent distressing psychological symptoms following sudden, threatening, or catastrophic events. Globally, approximately 70% of individuals experience traumatic events at some point in their lives, and nearly 4% develop chronic PTSD. The core features of PTSD include exaggerated fear responses and persistent, uncontrollable, intrusive traumatic memories. Clinically, cognitive behavioral therapies based on fear extinction, such as exposure therapy, aim to alleviate patients' fear states and improve their adaptive capacities. However, fear extinction—which normally promotes adaptive responses in healthy populations—is severely impaired in PTSD patients. Individuals with PTSD often fail to transition effectively from high to low fear responses through extinction training, or they relapse uncontrollably to heightened fear, thereby exacerbating core PTSD symptoms such as emotional disturbances, social avoidance, and cognitive dysregulation. Consequently, unraveling the neural mechanisms underlying impaired fear extinction in PTSD and developing novel interventions to facilitate fear extinction offer critical therapeutic opportunities for treating this disorder.
On September 24, 2024, a collaborative study led by Prof. Wei-Guang Li from our institute, Prof. Tian-Le Xu from Shanghai Jiao Tong University School of Medicine, and Prof. Ti-Fei Yuan from Shanghai Mental Health Center was published online in the prestigious international medical journal Journal of Clinical Investigation, titled "Stimulation of an entorhinal-hippocampal extinction circuit facilitates fear extinction in a post-traumatic stress disorder model." This study identifies a previously unknown neural circuit—from the lateral entorhinal cortex (LEC) to ventral hippocampal CA1 region (vCA1)—that plays a crucial role in fear extinction. It demonstrates that fear extinction recruits synchronized low-gamma (low-γ) oscillations between LEC and vCA1 as an intrinsic electrophysiological signature. Furthermore, employing clinically accessible techniques including deep brain stimulation (DBS) and non-invasive transcranial alternating current stimulation (tACS) to target the LEC→vCA1 circuit significantly enhanced fear extinction in mouse models. These findings provide novel theoretical foundations and robust preclinical evidence supporting precision therapies for PTSD and related extinction-resistant disorders.

Using multisite in vivo electrophysiological recordings, the collaborative team first demonstrated that fear extinction training specifically engages synchronized low-γ oscillations between LEC and vCA1. Further investigation using c-Fos immunostaining combined with fiber photometry recordings revealed significantly elevated activity of parvalbumin-positive (PV) interneurons in vCA1 during the late stages of extinction, mediating extinction-dependent low-γ synchronization between LEC and vCA1. Employing retrograde monosynaptic tracing, intersectional genetics, and electrophysiology, researchers discovered that vCA1 PV interneurons receive substantial direct excitatory inputs originating from Sim1-expressing fan cells in LEC layer 2a, forming direct monosynaptic connections. Optogenetic and chemogenetic manipulations demonstrated bidirectional modulation of fear extinction memory by selectively activating or inhibiting this LEC→vCA1 projection, accompanied by corresponding enhancement or reduction of LEC-vCA1 low-γ synchronization. Thus, the neural connection between LEC fan cells and vCA1 PV interneurons regulates fear extinction by orchestrating low-γ oscillatory synchronization.
To facilitate clinical translation, researchers developed a mouse model utilizing DBS targeting vCA1 with low-γ frequency stimulation. Low-γ DBS significantly activated PV interneurons in vCA1, enhanced fear extinction, and notably maintained improved extinction even one day after stimulation ceased. Mechanistically, low-γ DBS in vCA1 sustainably increased PV interneuron activity and enhanced feed-forward inhibition within the LEC-vCA1 circuit, effectively suppressing fear memory engram cells in vCA1 and augmenting fear extinction. Additionally, researchers developed a tACS protocol targeting LEC, achieving similar enhancements in fear extinction. Crucially, applying LEC-tACS in a mouse PTSD model characterized by pronounced extinction deficits produced long-lasting improvements in extinction and attenuated fear responses. These results indicate that non-invasive neural modulation targeting the LEC→vCA1 circuit effectively facilitates fear extinction, offering new clinical opportunities for PTSD treatment (see schematic figure).
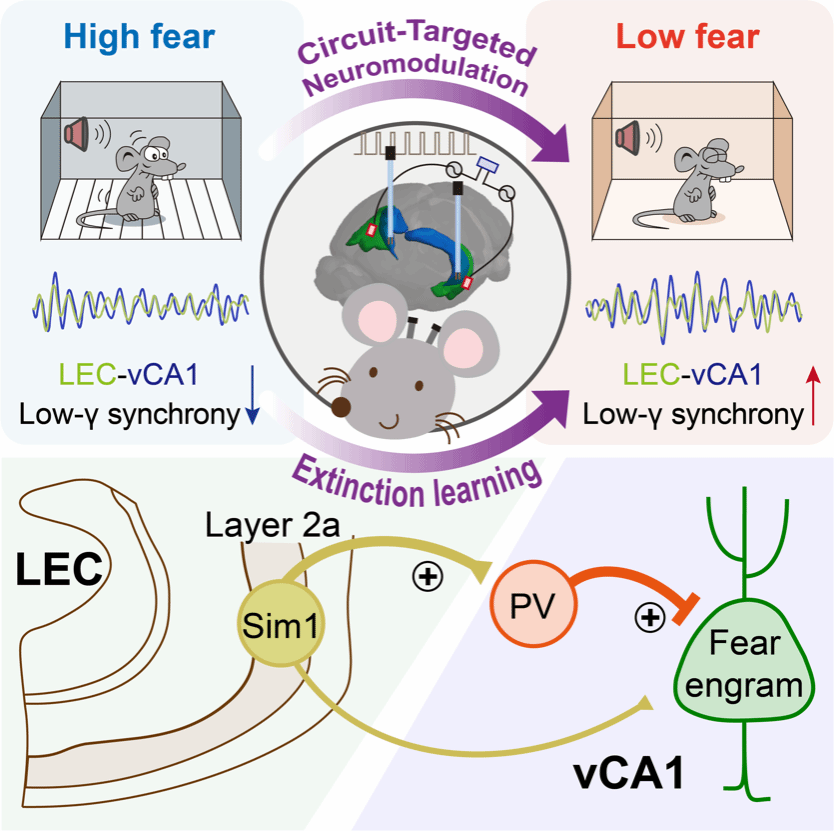
Figure. Neural circuit oscillatory signatures of fear extinction and targeted neuromodulation strategies.
Authorship and Acknowledgments:
Dr. Ze-Jie Lin (doctoral candidate, Shanghai Jiao Tong University School of Medicine) and Dr. Xue Gu (Department of Anesthesiology, Shanghai General Hospital) are co-first authors of this publication. Prof. Wei-Guang Li from our institute, Prof. Tian-Le Xu from Shanghai Jiao Tong University School of Medicine, and Prof. Ti-Fei Yuan from Shanghai Mental Health Center serve as co-corresponding authors.
This work was supported by funding from the Science and Technology Innovation 2030 Major Project ("Brain Science and Brain-inspired Research"), the National Natural Science Foundation of China, and various Shanghai municipal-level projects. The research also received collaborative support from Prof. Quanying Liu at Southern University of Science and Technology, Academician Lu-Yang Wang at the University of Toronto, and Prof. Michael X. Zhu at the University of Texas Health Science Center at Houston.
Link to original paper: https://doi.org/10.1172/JCI181095