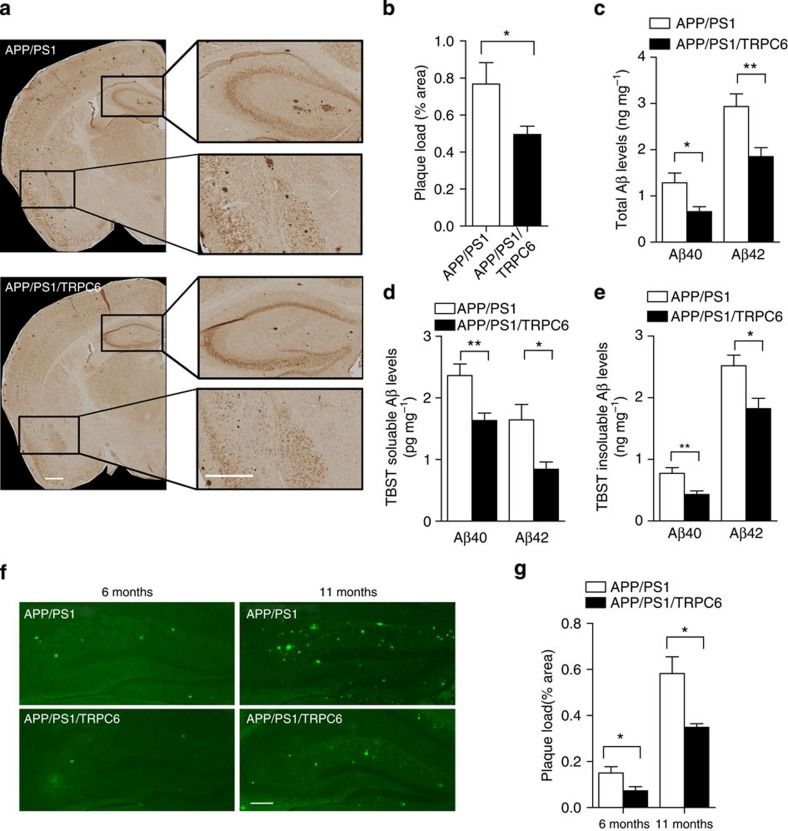
This study, entitled "TRPC6 specifically interacts with APP to inhibit its cleavage by γ-secretase and reduce Aβ production," was published online in Nature Communications in November 2015. The findings demonstrate that TRPC6 binds to APP, thereby blocking γ-secretase from binding to and proteolytically processing its substrate C99, which ultimately reduces Aβ generation. Notably, TRPC6 does not suppress the overall enzymatic activity of γ-secretase and has no inhibitory effect on the cleavage of other γ-secretase substrates, such as Notch, E-cadherin, or N-cadherin. Furthermore, short peptides derived from TRPC6 selectively inhibit γ-secretase-mediated APP processing and Aβ production without interfering with Notch cleavage. These results suggest that disrupting γ-secretase–APP interactions may represent a novel therapeutic strategy for Alzheimer's disease (AD). Consequently, identifying molecules that mimic the functional properties of TRPC6 or its peptide fragments could lead to innovative AD treatments targeting substrate-specific γ-secretase modulation.