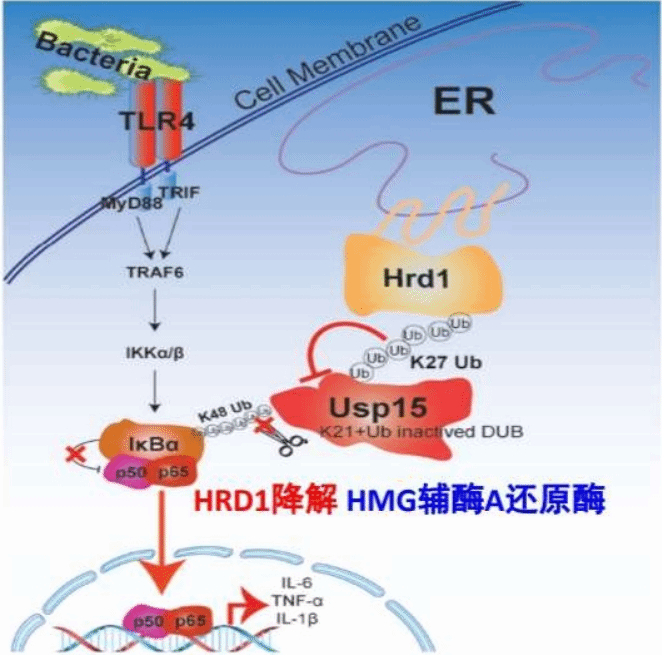
This study is shown in above panel titled as “The ER-localised Hrd1 ubiquitinates and inactivates Usp15 to promote TLR4-induced inflammation during bacterial infection”. Here, we screened more than 280 E3 ubiquitin ligases and discovered that the endoplasmic reticulum-located Hrd1 regulates TLR4-induced inflammation during bacterial infection. Hrd1 interacts directly with the deubiquitinating enzyme Usp15. Unlike the classical function of Hrd1 in endoplasmic reticulum-associated degradation, Usp15 is not degraded but loses its deubiquitinating activity for IκBα deubiquitination, resulting in excessive NF-κB activation. Importantly, Hrd1 deficiency in macrophages protects mice against lipopolysaccharide-induced septic shock, and knockdown of Usp15 in Hrd1-knockout macrophages restores the reduced IL-6 production. This study proposes that there is crosstalk between Hrd1 and TLR4, thereby linking the endoplasmic reticulum–plasma membrane function during bacterial infection.