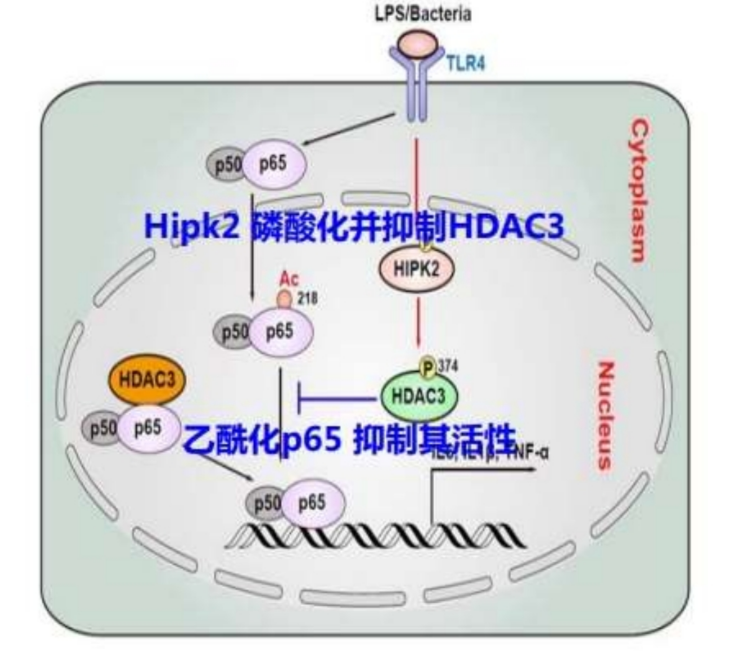
The study is one of our findings associate with “HIPK2 phosphorylates HDAC3 for NF-κB acetylation to ameliorate colitis-associated colorectal carcinoma and sepsis”. Here, we have identified that homeodomain interacting protein kinase 2 (HIPK2), a nuclear serine/threonine kinase, increases its expression in inflammatory macrophages. Importantly, HIPK2 deficiency or overexpression could enhance or inhibit inflammatory responses in LPS-stimulated macrophages, respectively. HIPK2-deficient mice were more susceptible to LPS induced endotoxemia and CLP-induced sepsis. Mechanistically, HIPK2 bound and phosphorylated histone deacetylase 3 (HDAC3) at serine 374 to inhibit its enzymatic activity, thus reducing the deacetylation of p65 at lysine 218 to suppress NF-κB activation. Our findings suggest that the HIPK2- HDAC3-p65 module in macrophages restrains excessive inflammation, which may represent a new layer of therapeutic mechanism for colitis-associated colorectal cancer and sepsis.